Students can Download Chapter 7 Equilibrium Questions and Answers, Plus One Chemistry Chapter Wise Questions and Answers helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 7 Equilibrium
Plus One Chemistry Equilibrium One Mark Questions and Answers
Question 1.
Equilibrium in a system having more than one phase is called _________
Answer:
heterogeneous
Question 2.
Addition of a catalyst to a chemical system at equilibrium would result in
a) Increase in the rate of forward reaction
b) Increase in the rate of reverse reaction
c) A new reaction path
d) Increase in the amount of heat evolved in the reaction
Answer:
c) A new reaction path
Question 3.
With increase in temperature, equilibrium constant of a reaction
a) Always increases
b) Always decreases
c) May increase or decrease depending upon the number of moles of reactants and products
d) May increase or decrease depending upon whether reaction is exothermic or endothermic
Answer:
d) May increase or decrease depending upon whether reaction is exothermic or endothermic
Question 4.
Water is a conjugate base of ____________ .
Answer:
H3O+
![]()
Question 5.
Which of the following substances on dissolving in water will give a basic solution?
a) Na2CO3
b) Al2(SO4)3
c) NH4Cl
d) KNO3
Answer:
a) Na2CO3
Ged exam books to download, equilibrium concentration calculator, Finding the Least Common Denominator, combining like expressions.
Question 6.
Choose the correct answer for the reaction,
N2(g) + 3H2(g) \(\rightleftharpoons \) 2NH3(g); ∆rH = -91.8 kJ mol-1 The concentration of H2(g) at equilibrium can be increased by
1) Lowering the temperature
2) Increase the volume of the system.
3) Adding N2 at constant volume.
4) Adding H2 at constant volume.
Answer:
2) and 4) are correct.
Question 7.
Conjugate base of a strong acid is a
Answer:
Weak base
Question 8.
The expression forostwald dilution law is
Answer:
Ka = Cα²
Question 9.
The hydroxyl ion concentration in a solution having pH = 4 will be
Answer:
10-14
Question 10.
A mono protic acid in 1M solution is 0.01 % ionized the dissociation constant of this acid is
Answer:
10-8
Question 11.
A certain buffer solution contains equal concentration of x– and Hx. The Kafor Hx is 10-6 pH of buffer is
Answer:
6
Question 12.
In the equilibrium reaction
CaCO3(s) → CaO(s) + CO2(g) the equilibrium constant is given by —–
Answer:
PCO2
Question 13.
Congugate base of a strong acid is a _________ .
a) Strong base
b) Strong acid
c) Weak acid
d) Weak base
e) Salt
Answer:
d) Weak base
Question 14.
The species acting both as bronsted acid and base is _________ .

Answer:
b) HSO4–
Question 15.
PH of .01 M KOH solution will be _________ .
Answer:
12
Plus One Chemistry Equilibrium Two Mark Questions and Answers
Question 1.
“High pressure and low temperature favours the formation of ammonia in Haber’s process.” Analyse the statement and illustrate the conditions using Le-Chatliers principle?
Answer:
The given statement is correct.
N2(g) + 3H2(g) \(\rightleftharpoons \) 2NH3(g); ∆rH =-91.8 kJ mol-1 Since the number of moles decreases in the forward reaction a high pressure of ~ 200 atm is applied. Since the forward reaction is exothermic the optimum temperature of ~ 700 K is employed for maximum yield of ammonia.
Question 2.
“Chemical equilibrium is dynamic in nature”. Analyse the statement and justify your answer.
Answer:
At equilibrium the reaction does not stop. Both forward and backward reactions are taking place at equal rates. Thus, at equilibrium two exactly opposite changes occur at the same rate. Hence, chemical equilibrium is dynamic in nature.
Question 3.
Pressure has no influence in the following equilibrium: N2(g) + O2(g) \(\rightleftharpoons \) 2NO(g)
- Do you agree with this?
- What is the reason for this?
Answer:
- Yes.
- Here the total number of moles of the reactants is equal to that of the products. Hence pressure is having no influence in this equilibrium.
Question 4.
During a class room discussion a student is of the view that the value of equilibrium constant can be influenced by catalyst.
- Do you agree with the statement?
- Justify the role of catalyst in an equilibrium reaction?
Answer:
- No.
- Catalyst does not affect the equilibrium composition of a reaction mixture. It does not appear in the balanced chemical equation or in the equilibrium constant expression. It only helps to attain the equilibrium state in a faster rate.
Question 5.
What is the equilibrium constant (K) in the following cases?
- Reaction is reversed.
- Reaction is divide by 2.
- Reaction is multiplied by 2.
- Reaction is splitted into two.
Answer:
- 1/K
- √K
- K2
- K1K2
Question 6.
1. What is homogeneous equilibrium?
2. Suggest an example for this.
Answer:
1. The equilibrium in which the reactants and products are in the same phase,
2. N2(g) + 3H2(g) \(\rightleftharpoons \) 2NH3(g)
In this equilibrium, the reactants and products are in the gaseous phase.
![]()
Question 7.
The equilibrium constants for two reactions are given. In which case the yield of product will be the maximum?
For first reaction: K1 = 3.2 × 10-6
For second reaction: K2 = 7.4 × 10-6
Answer:
Higher the value of K, greater will be the yield of product. So maximum yield will be in the second case.
Question 8.
Write an expression for equilibrium constant, Kc forthe ‘ reaction, 4NH3(g) + 5O2(g) \(\rightleftharpoons \) 4NO(g) + 6H2O(g)
Answer:
![]()
Question 9.
1. State Henry’s law.
2. Suggest an example fora gas in liquid equilibrium.
Answer:
1. The mass of a gas dissolved in a given mass of a solvent at any temperature is directly proportional to the pressure of the gas above the solvent.
2. Equilibrium between the CO2 molecules in the. gaseous state and the CO2 molecules dissolved in water under pressure,
CO2(g) \(\rightleftharpoons \) CO2(in solution)
Question 10.
1. What is heterogeneous equilibrium?
2. Suggest an example forthis.
Answer:
1. Equilibrium in a system having more than one phase is called heterogeneous equilibrium
2. Equilibrium between solid Ca(OH)2 and its saturated solution:
Ca(OH)2(s) + (aq) \(\rightleftharpoons \) Ca2+(aq) + 2OH–(aq)
Question 11.
For the equilibrium 2SO3(g) → 2SO2(g) + O2(g), Kc at 47 °C 3.25 × 10-9 mol per litre. What will be the value of Kp at this temperature (R = 8.314 J K-1mol-1).
Answer:
R = 8.314 J K-1 mol-1 ∆n = 3 – 2 = 1
T = 47 °C = 273 + 47 = 320 K
Kc = 3.25 × 10-9
Kp = Kc (RT)∆n
= 3.25 × 10-9 (8.314 × 320)1
= 3.25 × 10-9 × 8.314 × 320 = 8.65 × 10-12
Question 12.
The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ions in it.
Answer:
pH= -log[H+] = 3.76
log[H+] = – 3.76
[H+] = antilog of (- 3.76) = 1.738 × 10-4 mol L-1
Question 13.
The equilibrium constant can be expressed in terms of partial pressure as well as concentration.
1. Give the relation between Kp and Kc.
2. What is the relation between Kp and Kc for the reaction, N2(g) + O2(g) \(\rightleftharpoons \) 2NO(g)?
Answer:
1. Kp = Kc(RT)∆n, where ∆n = (number of moles of gaseous products) – (number of moles of gaseous reactants).
2. Here, ∆n = 2 – 2 = 0
Kp = Kc(RT)∆n , Kp = Kc (RT)°
∴ Kp = Kc
Question 14.
1. Explain Arrhenius concept of acids and bases with suitable examples.
2. How proton exists in aqueous solution? Give reason.
Answer:
1. According to Arrhenius theory, acids are substances that dissociates in water to give hydrogen ions, H+(aq) and bases are substances that produce hydroxyl ions, OH–(aq). Forexample, HCl is an Arrhenius acid and NaOH is an Arrhenius base.
2. In aqueous solution the proton bonds to the oxygen atom of a solvent water molecule to give trigonal pyramidal hydronium ion, H3O+(aq). This is because a bare proton, H+ is very reactive and cannot exist freely in aqueous solutions.
Question 15.
1. What is an acidic buffer?
2. Suggest an example for an acidic buffer.
Answer:
1. An acidic buffer is a buffer solution having pH less than 7. It is prepared by mixing a weak acid and its salt formed with a strong base.
2. Mixture of acetic acid (CH3COOH) and sodium acetate (CH3COONa) is an example for an acidic buffer. Its pH is around 4.75.
Question 16.
1. What is a basic buffer?
2. Suggest an example for basic buffer.
Answer:
1. A basic buffer is a buffer solution having pH greater than 7. It is prepared by mixing a weak base and its salt formed with a strong acid.
2. Mixture of ammonium hydroxide (NH4OH) and ammonium chloride (NH4CI) is an example for a basic buffer. Its pH is around 9.25.
Plus One Chemistry Equilibrium Three Mark Questions and Answers
Question 1.
The concentration of reactant and products for the reaction, H2(g) + l2(g) \(\rightleftharpoons \) 2Hl(g) are recorded as follows:
| Reactant or Product | Molar Concentration |
| H2 | 0.080 |
| l2 | 0.060 |
| HI | 0.490 |
a) Write down the expressions for equilibrium constant of the above reaction.
b) Calculate the equilibrium constant at the temperature 298 K if [Hl] = 0.49 M, [H2]=0.08 M and [l2]=0.06 M.
Answer:

Question 2.
1. When equilibrium is reached in a chemical reaction?
2. What is the influence of molar concentration in a reaction at equilibrium?
3. Write the expression for equilibrium constant for the decomposition of NH4CI by the reaction,
NH4Cl \(\rightleftharpoons \) NH3+HCl
Answer:
1. When the rate of forward reaction is equal to rate of backward reaction, the chemical reaction is said to be in equilibrium.
2. Rate of chemical reaction is directly proportional to the product of molar concentration of the reactants.
3. \(\mathrm{K}=\frac{\left[\mathrm{NH}_{3}\right] \mathrm{HCl}}{\left[\mathrm{NH}_{4} \mathrm{Cl}\right]}\)
![]()
Question 3.
1. What is meant by Kp?
2. How Kp is related to Kc?
Answer:
1. Kp is the equilibrium constant in terms of the partial pressures of the reactants and products (Pressure should be expressed in bar as standard state is 1 bar). It is used for reactions involving gases.
2. Kp = Kc (RT)∆n
where R = universal gas constant, T = absolute temperature and ∆n = number of moles of gaseous product(s) – number of moles of gasesous reactant(s).
Question 4.
a) What do you mean by equilibrium constant?
b) Write any two characteristics of equilibrium constant.
c) Write an expression for equilibrium constant of the reaction, 2SO2(g) + O2(g) \(\rightleftharpoons \) SO3(g).
Answer:
a) Equilibrium constant at a given temperature is the ratio of product of molar concentrations of the products to that of the reactants, each concentration term being raised to the respective stoichiometric coefficients in the balanced chemical equation.
b) 1. The value of equilibrium constant is independent of the initial concentrations of the reactants and products.
2. Equilibrium constant is temperature dependent having one unique value for a particular reaction represented by a balanced equation at a given temperature.
Question 5.
2NO2(g) \(\rightleftharpoons \) N2O4(g); ∆H = -52.7 kJ mol-1
1. What change will happen if we increase the temperature?
2. What is the effect of increase in pressure in the above equilibrium?
3. What happens when N2O4 is removed from the reaction medium?
Answer:
1. Since the forward reaction is exothermic, on increasing temperature the rate of backward reaction (endothermic reaction) increases.
2. Since the number of moles decreases in the forward reaction, on increasing pressure, the rate of forward reaction increases.
3. Rate of forward reaction increases.
Question 6.
Consider this reaction:
CO(g) + 2H2(g) \(\rightleftharpoons \) CH3OH(g); ∆rH = -92 kJ mol-1
Explain the influence of the following on the basis of
Le Chatelier’s principle.
1. Decrease in pressure.
2. Increase in temperature.
3. Increase in the partial pressure of hydrogen.
Answer:
1. On decreasing pressure the reaction shifts in the direction in which there is increase in the number of moles. Thus, the rate of backward reaction increases on decreasing pressure.
2. On increasing temperature, the rate of endothermic reaction increases. Here, backward reaction is endothermic. Hence, on increasing temperature the rate of backward reaction increases.
3. Hydrogen, being a reactant increase in its partial pressure increases the rate of forward reaction.
Question 7.
The equilibrium showing dissociation of phosgene gas is given below:
COCl2(g) \(\rightleftharpoons \) CO(g) + Cl2(g)
When a mixture of these three gases at equilibrium is compressed at constant temperature, what happens to
1. The amount of CO in mixture?
2. The partial pressure of COCl2?
3. The equilibrium constant for the reaction?
Answer:
1. Amount of CO decreases, because the system favours the reaction in which number of moles decreases with increase of pressure i.e., backward reaction.
2. Increases.
3. Equilibrium constant remains the same since temperature is constant.
Question 8.
The equilibrium constant of the reaction H2(g) + l2(g) \(\rightleftharpoons \) 2Hl(g) is 57 at 700 K. Now, give the equilibrium constants for the following reactions at the same temperature:

Answer:

Question 9.
1. What are buffer solutions?
2. Which of the following are buffer solutions?
NaCl + HCl
NH4Cl + NH4OH
HCOOH + HCOOK
3. What is the effect of pressure on the following equilibria?
i) Ice \(\rightleftharpoons \) Water
ii) N2(g) + O2(g) \(\rightleftharpoons \) 2N0(g)
Answer:
1. These are solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali.
2. NH4Cl + NH4OH
3. i) When pressure is increased the melting point of ice decreases and hence the rate of forward reaction will increase.
ii) Pressure has no effect in this equilibrium because there is no change in the number of moles of the gaseous reactants and products.
Question 10.
The aqueous solution of the compounds NaCl, NH4Cl and CH3COONa show different pH.
- Identify the acidic, basic and neutral solution among them.
- The concentration of hydrogen ion in a soft drink is 4 × 10-4. What is its pH?
Answer:
- Acidic-aqueous solution of NH4Cl Neutral – aqueous solution of NaCl Basic – aqueous solution of CH3COONa
- pH = – log[H+] = – log[4 × 10-4] = 3.398
Question 11.
1. What is pH? What is its significance?
2. The concentration of hydrogen ion in a sample of soft drink is 3.8 × 10-3. What is its pH?
Answer:
1. pH is a logarithmic scale used to express the hydronium ion concenration in molarity more conveniently. The pH of a solution is defined as negative logarithm to the base 10 of the activity of hydrogen ion. pH = — log \({ { a }_{ { H }^{ + } } }\) = —log[H+]
2. [H+] = 3.8 × 10-3
pH = -log[H+]
= -log [3.8 × 10-3] = -(-2.42) = 2.42
Question 12.
1. State the Le-Chatelier’s principle.
2. Apply the above principle in the following equilibrium and predict the effect of pressure.
CO(g) + 3H2(g) \(\rightleftharpoons \) CH4(g) + H2O(g)
Answer:
1. The Le Chateliers principle states that a change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change.
2. On increasing pressure the rate of forward reaction increases. This is because number of moles decreases in the forward reaction. In other words, the value of Qc decreases on increasing pressure. As Qc < Kc, the reaction proceeds in the forward direction.
![]()
Question 13.
1. Explain Lewis concept of acids and bases.
2. Why does BF3 act as a Lewis acid?
Answer:
1. A Lewis acid can be defined as a species which accepts electron pair and a Lewis base is a species which donates an electron pair.
2. In BF3, the boron atom is electron deficient and it accepts a lone pair of electron. So it acts as a Lewis acid.
Question 14.
1. How the value of AG influence the direction of an equilibrium process?
2. The equilibrium constant for a reaction is 8. What will be the value of ∆G at 27 °C?
Answer:
1. If ∆G is negative, then the reaction is spontaneous and proceeds in the forward direction.
If ∆G is positive, then reaction is considered non- spontaneous. instead, as reverse reaction would have a negative ∆G, the products of the forward reaction shall be converted to the reactants.
If ∆G is 0, reaction has achieved equilibrium. At this point, there is no longer any free energy to drive the reaction.
2.

Question 15.
1. What is common ion effect?
2. Suggest an example for this effect.
Answer:
1. Common ion effect may be defined as the suppression of the dissociation of a weak electrolyte by the addition of some strong electrolyte containing a common ion.
2. The dissociation equilibrium of NH4OH is shifted towards left in presence of NH4Cl having the common ion, NH4+.
Question 16.
1. Predict whether an aqueous solution of (NH4)2SO4 is acidic, basic or neutral?
2. Justify your answer.
Answer:
1. An aqueous solution of (NH4)2SC4 is acidic in nature.
2. (NH4)2SO4 is formed from weak base, NH4OH, and strong acid, H2SO4. In water, it dissociates completely
(NH4)2SO4(aq) → 2NH4+ (aq) + SO42- (aq)
NH4+ ions undergoes hydrolysis to form NH4OH and H+ ions.
NH4+(aq) + H2O(l) \(\rightleftharpoons \) NH4OH(aq) + H+(aq)
NH4OH is a weak base and therefore remains almost unionised in solution. This results in increased H+ ion concentration in solution making the solution acidic.
Question 17.
1. What are sparingly soluble salts? Suggest an example.
2. Define solubility product constant, Ksp.
3. Obtain the relation between solubility product constant (Ksp) and solubility (S), of a solid salt of general formula Mxp+ Xyq-.
Answer:
1. Sparingly soluble salts are those salts with solubility less than 0.01 M.
e.g. BaSO4
2. The solubility product of a sparingly soluble salt at a given temperature is defined as the product of the concentrations of its ions in the saturated solution, with each concentration term raised to the power equal to the number of times the ion occurs in the equation representing the dissociation of the electrolyte.
3. The equilibrium in the saturated solution of the salt can be represented as,

Question 18.
The Le Chatelier’s principle is applicable to physical and chemical equilibria.
1. What are the factors which can influence the equilibrium state of a system?
2. Explain the factors affecting the chemical equilibrium on the basis of Le Chatelier’s principle taking Haber’s process for the manufacture of ammonia as an example.
Answer:
1. The following factors can influence the equilibrium state of a system:
- Change in concentration of the reactants or products.
- Change in temperature.
- Change in pressure.
- Addition of inert gas.
- Presence of catalyst.
2. N2(g) + 3H2(g) \(\rightleftharpoons \) 2NH3(g); ∆rH = -91.8 kJ mol-1
When concentration of N2 or H2 is increased, a good yield of NH3 can be achieved. The rate of forward reaction can also be increased by removing NH3 from the reaction mixture.
When pressure is increased, the system will try to decrease pressure and for this system will proceed in that direction where there is minimum number of moles i.e., forward reaction. Thus, a good yield of NH3 can be achieved by increasing pressure.
Since the formation of NH3 is an exothermic reaction, a good yield of NH3 can be achieved by decreasing the temperature. But if the temperature is decreased to very low value the reactant molecules do not have sufficient energy to interact. Hence, an optimum temperature of 500°C is used.
![]()
Question 19.
1. Soda water is prepared by dissolving CO2 in water under high pressure. What is the principle involved in this process?
2. At 1000 K, equilibrium constant Kc for the reaction 2SO3(g) \(\rightleftharpoons \) 2SO2(g) + O2(g) is 0.027. What is the value of Kp at this temperature?
Answer:
1. Henry’s law
2. Kp =Kc(RT)∆n
∆n = 3.2 = 1
Kp = 0.027 × (0.0831 × 1000)1 = 2.2437
Question 20.
1. For the reaction PCL \(\rightleftharpoons \) PCl3 +Clc
i) Write the expression of Kc.
ii) What happens if pressure is increased?
2. Write the conjugate acid and base of the following species:
i) H20 ii) HCO;
3. Name the phenomenon involved in the preparation of soap by adding NaCI.
Answer:
1. i) \(\kappa_{c}=\frac{\left[\mathrm{PC}_{3}\right]\left[\mathrm{Cl}_{2}\right]}{\left[\mathrm{PCl}_{5}\right]}\)
ii) If we increase the pressure the system will try to decrease the pressure. For this system will proceed in the direction where there is minimum number of moles, i.e., rate of backward reaction increases by decreasing the pressure.
2. i) Conjugate acid of H2O is H3O+
Conjugate base of H2O is OH”
ii) Conjugate acid of HCO,” is H2CO3 Conjugate base of HCO3– is CO32-
3. Common ion effect.
Question 21.
a) The pH of black coffee is 5.0. Calculate the hydrogen ion concentration.
b) The Ksp of barium sulphate is 1.5 × 10-9. Calculate the solubility of barium sulphate in pure water.
Answer:

Question 22.
1. What is conjugate acid-base pair?
2. Illustrate with an example.
Answer:
1. The acid-base pairthat differs only by one proton is called conjugate acid-base pair. Such acid-base pairs are formed by loss or gain of a proton.
2. Consider the ionization of hydrochloric acid in water.

HCl(aq) acts as an acid by donating a proton to H2O molecule which acts as a base because it accepts the proton. The species H2O+ is produced when water accepts a proton from HCl. Therefore, Cl– is the conjugate base of HCl and HCl is the conjugate acid of Cl–. Similarly, H2O is the conjugate base of H2O+ and H3O++ is the conjugate acid of the base H2O.
Question 23.
1. What are the applications of equilibrium constant?
2. What is meant by reaction quotient, Qc?
3. Predict the direction of net reaction in the following cases:
i) Qc < Kc
ii) Qc > Kc
iii) Qc = Kc
Answer:
1. The applications of equilibrium constant are:
• To predict the extent of a reaction on the basis of its magnitude.
• To predict the direction of the reaction.
• To calculate equilibrium concentrations.
2. Reaction quotient, Qc at a given temperature is defined as the ratio of the product of concentrations of the reaction products to that of the reactants, each concentration term being raised to their individual stoichiometric coefficients in the balanced chemical equation, where the concentrations are not necessarily equilibrium values.
3. i) When Qc > Kc, the reaction will proceed in the
direction of reactants (reverse reaction), i.e., net reaction goes from right to left.
ii) When Qc < Kc, the reaction will proceed in the direction of products (forward reaction), i.e., net reaction goes from left to right.
iii) When Qc = Kc, the reaction mixture will be at equilibrium, i.e., no net reaction occurs.
Question 24.
Solubility product helps to predict the precipitation of salts from solution.
1. Find the relation between solubility (S) and solubility product (Ksp) of calcium fluoride and zirconium phosphate.
2. The solubility product of two sparingly soluble salts XY2 and AB are 4 × 10-15 and 1.2 × 10-16 respectively. Which salt is more soluble? Explain.
Answer:
1. The equilibrium in the saturated solution of calcium fluoride can be represented as,
CaF2(s) \(\rightleftharpoons \) Ca2+(aq) + 2F–(aq)
Ksp = [Ca2+][F–]2 = S.(2S)2 = 4S3
The equilibrium in the saturated solution of zirconium phosphate can be represented as,
Zr3(PO4)4(s) \(\rightleftharpoons \) 3Zr4+(aq) + 4PO43-(aq)
Ksp = [Zr4+]3[PO43-]4 = (3S)3.(4S)4 = 6912S7
2. XY2 is more soluble than AB.

Ionic Strength Calculator … Ionic strength of a solution indicates the concentration of ionic charge in the solution.
Question 25.
a) How common ion effect can influence the solubility of ionic salts?
b) What is the application of common ion effect in gravimetric estimation?
Answer:
1. In a salt solution, if we increase the concentration of any one of the ions, according to Le Chatelier’s principle, it should combine with the ion of its opposite charge and some of the salt will be precipitated till Ksp = Qsp. Similarly, if the concentration of one of the ions is decreased, more salt will dissolve to increase the concentration of both the ions till Ksp = Qsp.
2. The common ion effect is used for almost complete precipitation of a particular ion as its sparingly soluble salt, with very low value of solubility product for gravimetric estimation.
Plus One Chemistry Equilibrium Four Mark Questions and Answers
Question 1.
Match the following:

Answer:

Question 2.
In Contact process, SO3 is prepared by the oxidation of SO2 as per the following reaction:
2SO2(g) + O2(g) \(\rightleftharpoons \) 2SO3(g); ∆H = -189.4
a) What happens to the rate of forward reaction when i) temperature is increased?
ii) pressure is decreased?
iii) a catalyst V2O5 is added?
b) Calculate the pH of 0.01 M H2SO4 solution. Also, calculate the hydroxyl ion concentration in the above solution.
Answer:
1. D When temperature is increased, the rate of forward reaction decreases since it is exothermic.
ii) When pressure is decreased the rate of forward reaction decreases since it is associated with decrease in number of moles.
iii) When a catalyst V2O5 is added the rate of both forward and backward reactions are increased by the same extent and equilibrium is reached earlier.
2.

The method presented in our buffer pH calculator allows you to compute the pH of both arterial and venous blood.
Question 3.
Calculate the [H+] in the following biological fluids whose pH are given in brackets.
i) Human muscle fluid (6.83)
ii) Human stomach fluid (1.22)
iii) Human blood (7.38)
iv) Human saliva (6.4)
Answer:
i) pH =-log[H+] = 6.83
log[H+] = – 6.83
[H+] = antilog (- 6.83) = 1.48 × 10-7 mol L-1
ii) [H+] = antilog (- 1.22) = 6.03 × 10-2 mol L-1
iii) [H+] = antilog (- 7.38) = 4.17 × 10-8 mol L-1
iv) [H+] = antilog (- 6.4) = 3.98 × 10-7 mol L-1
Question 4.
The pH value of a solution determines whether it is acidic, basic or neutral in nature.
1. The concentration of hydrogen ion in the sample of a soft drink is 3.8 × 10-3 mol/L. Calculate its pH. Also predict whether the above solution is acidic, basic or neutral.
2. The dissociation constants of formic acid (HCOOH) and acetic acid (CH3COOH) are 1.8 × 10-4and 1.8 × 10-4 respectively. Which is relatively more acidic? Justify your answer.
Answer:
1. pH = – log[H+] = – log[3.8 × 10-3] = 2.42
Since pH is less than 7, it is an acidic solution,
2. HCOOH is more acidic.
Ka value is directly proportional to the acid strength, i.e., greater the Ka value, stronger is the acid.
Question 5.
a) Write the expression for Henderson – Hasselbalch equation for i) An acidic buffer & ii) A basic buffer.
b) Calculate the pH of a solution which is 0.1 M in
CH3COOH and 0.5 M in CH3COONa. Ka for CH3COOH is 1.8 × 10-6.
Answer:

Plus One Chemistry Equilibrium NCERT Questions and Answers
Question 1
A liquid is in equilibrium with its vapour in a sealed containerat a fixed temperature. The volume of the container is suddenly increased. (3)
a) What is the initial effect of the change on vapour pressure?
b) How do rates of evaporation and condensation change initially?
c) What happens when equilibrium is restored finally and what will be the final vapour pressure?
Answer:
a) Vapour pressure decreases due to increase in volume.
b) Rate of evaporation remains same and rate of condensation decreases.
c) Finally the same vapour pressure is restored and the rate of evaporation becomes equal to the rate
Question 2.
The concentration of hydrogen ion in a sample of soft drink is 3.8 × 10-3 M. What is its pH? (2)
Answer:
pH = -log[H+]
= -log (3.8 × 10-3) = 2.42
![]()
Question 3.
The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it. (2)
Answer:
H= -log [H+]
or log [H+] = -3.76 = -4.24
[H+] = antilog (-3.76) = 1.74 × 10-4M
Question 4.
The ionization constants of HF, HCOOH and HCN at 298 K are 6.8 × 10-4, 1.8 × 10-4 and 4.8 × 10-9 respectively. Calculate the ionization constants of the corresponding conjugate base. (3)
Answer:
The relation between ionization constant of an acid and that of its conjungate base is Ka x Kb = Kw

Question 5
The ionization constant of nitrous acid is 4.5 x 10-4. Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis. (2)
Answer:
Sodium nitrite is a salt of strong base and weak acid. Its degree of hydrolysis, h is given by the relation.
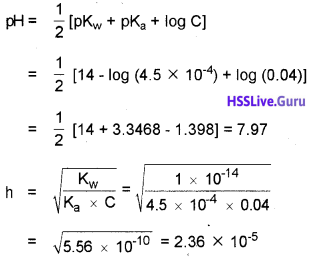
Question 6
A 0.02 M solution of pyridinium hydrochloride has pH = 3.44. Calculate the ionization constant of pyridine. (2)
Answer:
Pyridinium hydrochloride is a salt of a weak base (pyridine) and a strong acid (HCl). The pH of an aqueous solution of this salt is given by the relation:
