Students can Download Chapter 8 Redox Reactions Questions and Answers, Plus One Chemistry Chapter Wise Questions and Answers helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 8 Redox Reactions
Plus One Chemistry Redox Reactions One Mark Questions and Answers
Question 1.
In which of the following, oxidation number of chlorine is +5?
a) Cl–
b) ClO–
c) ClO2–
d) ClO3–
Answer:
d) ClO3–
Question 2.
An oxidising agent is a substance which can
a) Gain electrons
b) Lose an electronegative radical
c) Undergo decrease in the oxidation number of one of its atoms
d) Undergo any one of the above changes
Answer:
d) Undergo any one of the above changes
Question 3.
The arrangement of metals in the order of decreasing tendency to lose electrons is called _________ .
Answer:
Activity series
![]()
Question 4.
When KMnO4, reacts with acidified FeSO4
a) Only FeSO4 is oxidised
b) Only KMnO4 is oxidised
c) FeSO4 is oxidised and KMnO4 is reduced
d) KMnO4 is oxidised and FeSO4 is reduced
Answer:
c) FeSO4 is oxidised and KMnO4 is reduced
Question 5.
In the disproportionation reaction, which of the following statements is not true?
a) The same species is simultaneously oxidised as well as reduced
b) The reacting species must contain an element having at least three oxidation states
c) The element in the reacting species is present in the lowest oxidation state
d) The element in the reacting species is present in the intermediate oxidation state
Answer:
c) The element in the reacting species is present in the lowest oxidation state
Question 6.
Find the oxidation state of oxygen in OF2.
Answer:
The oxidation number of fluorine in its compounds is always taken as -1.
In OF2
X+ (-1 × 2) = 0
X = +2
Question 7.
The oxidation numbers of chlorine atoms in bleaching powder is _________ .
Answer:
-1
Question 8
SO2 can act as
a) Oxidising agent only
b) Reducing agent only
c) Both oxidising and reducing agents
d) Acid and a reducing agent only
Answer:
c) Both oxidising and reducing agents
Question 9.
In the reaction
2KMnO4 +16HCl → 5Cl2 + MnCl2 + 2KCl + 8H2O the reduction product is _________ .
Answer:
MnCl2
![]()
Question 10.
The strongest reducing agent is .
a) K
b) Ba
c) Li
d) Na
Answer:
c) Li
Question 11.
Oxidation state of oxygen in H2O2 is _________ .
Answer:
-1
Plus One Chemistry Redox Reactions Two Mark Questions and Answers
Question 1.
Balance the following equation using oxidation number method:
MnO2 + Cl– → Mn2+ + Cl2
Answer:
Assigning oxidation numbers:
![]()
Equating the increase and decrease in oxidation number:
MnO2 + 2Cl– → Mn2+ + Cl2
Balancing hydrogen and oxygen atoms:
MnO2 + 2Cl– + 4H+ → Mn2+ + Cl2 + 2H2O
Question 2.
Balance the following equation using half reaction method:
Cu + NO3– → Cu2+ + NO2
Answer:
Separating into half reactions:
Oxidation half: Cu → Cu2+
Reduction half: NO3– → NO2
Balancing oxygen and hydrogen atoms:
NO3– + 2H+ → NO2 + H2O
Balancing charge by adding electrons and making the number of electrons equal in the two half reactions:
Cu → Cu2+ + 2e–
2NO3– + 4H+ + 2e– → 2NO2 + 2H2O
Adding the two half reactions to achieve the overall reaction:
Cu + 2NO3 + 4H+ → Cu2+ + 2NO2 + 2H2O
Question 3.
Complete the following ionic equations:
- Al3+ + 3e– → …………….
- MnO42- → + e–
- K → K+ + ……………
- Fe2+ → Fe3+ +
Answer:
- Al3+ + 3e → Al
- MnO42- → MnO4–+ e–
- K → K+ + e–
- Fe2+ → Fe3+ + e–
![]()
Question 4.
Find the oxidation number of P in the following compounds:
- Na2PO4
- H3P2O7
- PH3
- H3PO4
Answer:
- Na2PO4, Oxidation state of P = +6
- H4P2O7, Oxidation state of P = +5
- PH3, Oxidation state of P = -3
- H3PO4, Oxidation state of P = +5
Question 5.
Choose the correct oxidation number of sulphur in the compounds in column Afrom column B.
| Column A | Column B |
| Na2SO4 | -2 |
| H2sO3 | +7 |
| H2S | +6 |
| H2S2O7 | +4 |
Answer:
| Column A | Column B |
| Na2SO4 | +6 |
| H2SO3 | +4 |
| H2S | -2 |
| H2S2O8 | +7 |
Question 6.
Explain oxidation number and valency.
Answer:
Valency of an atom is its combining capacity and is denoted by a number without sign. The valency of an element is always a whole number.
Oxidation number is a net charge which an atom has or appears to have when the other atoms from the molecule are removed as ions assuming that the shared pair of electrons is with more electronegative atom.
Question 7.
Some rules related to oxidation number are given below. Correct the mistakes.
- Oxidation number of alkali metals and alkaline earth metals is +2.
- Oxidation number of hydrogen is always +1.
- Algebraicsum of oxidation number of all the atoms in an ion is not equal to the charge on the ion.
Answer:
- Oxidation number of alkali metals is +1.
- Oxidation number of alkaline earth metals is +2.
- Oxidation number of H is +1 except in metallic hydrides.
Question 8.
Match the following:
| Oxidation number of Cl in Cl2O7 | Cu |
| Oxidant | Zn |
| Stannous Chloride, SnCl2 | +7 |
| Oxidation number of C in diamond | Get reduced easily |
| The metal which can’t displace H from dil.HCl | Zero |
| Reducing agent for mercuric chloride |
Answer:
| Oxidation number of Cl in Cl2O7 | +7 |
| Oxidant | Get reduced I easily |
| Stannous Chloride, SnCl2 | Reducing agent for mercuric chloride |
| Oxidation number of C in diamond | Zero |
| The metal which can’t displace H from dil.HCl | Cu |
Question 9.
1. Calculate the oxidation number of oxygen in OF2 and KO2.
2. When Zn rod is dipped in blue CuSO4 solution ‘ the blue colour of CuSO4 fades due to displacement reaction. Write the reaction and identify the following:
i) The substance oxidised and the substance reduced.
ii) The oxidant and the reductant.
Answer:
1. OF2: x + (-1 × 2) = 0
x – 2 = 0
x = +2
KO2: (+1 × 1) + 2x = 0
1 + 2x = 0
2x = -1
x = –\(\frac{1}{2}\)
2. Zn(s) + CuSO4 (aq) → ZnSO4(aq) + Cu(s)
i) Substance oxidised – Zn
Substance reduced – Cu
ii) Oxidant-Cu
Reductant – Zn
![]()
Question 10.
a) Calculate the oxidation number of C in CH4 and in CH3Cl.
b) The sum of oxidation numbers of all atoms in a molecule is …………
Answer:
a) CH4:
x + (1 × 4) = 0
x + 4 = 0
x = -4
Oxidation number of C in CH4 is -4.
CH3Cl:
x + (3 × 1) + -1 = 0
x + 3 – 1= 0
x + 2 = 0
x = -2
Oxidation number of C in CH3Cl is -2.
b) Zero
Question 11.
1. Write the oxidation state of each element and identify the oxidising agent and reducing agent in the following reaction:
H2S(g) + Cl2(g) → 2HCl(g) + S(s)
2. Fill in the blanks and classify the following reactions into oxidation and reduction:
i) Mn7+ + 5e– → ……………
ii) Sn4+ + …………… → Sn2+
iii) Na → Na+ + ……………
iv) Fe3+ +…………… → Fe2+
Answer:
1.![]()
Reducing agent – H2S
Oxidising agent -Cl2
2. i) Mn7+ + 5e– → Mn2+
ii) Sn4+ + 2e– → Sn2+
iii) Na → Na+ + e–
iv) Fe3+ + e– → Fe2+
Oxidation: Reaction (iii)
Reduction: Reactions (i), (ii) and (iv)
Question 12.
Dihydrogen undergoes redox reactions with many metals at high temperature.
a) Write the reaction between hydrogen with sodium.
b) Comment, whether the product formed, is covalent compound or ionic compound.
c) Which is the reducing agent in this reaction?
Answer:
1. 2Na + H2 → 2NaH
2. Ionic compound is formed. When alkali metals react with hydrogen ionic hydrides are formed.
3. Na is the reducing agent.
![]()
Question 13.
1. Is it possible to keep copper sulphate solution in zinc pot? Why?
2. Assign oxidation numbers of the underlined elements.
i) NaH2\(\underline { P } \)O4
ii) NaH\(\underline { S } \)O4
Answer:
1. No. Zn being more reactive will displace Cu from CuSO4. Thus Cu will be deposited on the vessel.
2. i) NaH2\(\underline { P } \)O4
+1 +(+1 × 2) + x +(-2 × 4) = 0
1 + 2 + x – 8 = 0
x – 5 = 0
x = +5
ii) NaH\(\underline { S } \)O4
+1 + 1 + x +(-2 × 4) = 0
+1 + 1 + x – 8 = 0
x – 6 = 0
x = +6
Question 14.
Identify the substance oxidised, reduced, oxidising agent and reducing agent in the reaction:
2Cu2O + Cu2S → 6Cu + SO2
Answer:
![]()
In this reaction, Cu is reduced from +1 state to zero. oxidation state and S is oxidised from -2 state to +4 state. Cu2O helps S in Cu2S to increase its oxidation number. Therefore, Cu(l) is the oxidising agent. S of Cu2S helps Cu both in Cu2S itself and Cu2O to decrease its oxidation number. Therefore, S of Cu2S is the reducing agent.
Question 15.
Explain the following in terms of electron transfer concept:
- Oxidation
- Reduction
- Oxidising agent
- Reducing agent
Answer:
- Oxidation: Loss of electron(s) by any species.
- Reduction: Gain of electron(s) by any species.
- Oxidising agent: Any species which accepts electrons).
- Reducing agent: Any species which donates electron^).
Question 16.
Represent the following compounds using Stock notation:
Cu2O, SnCl4, MnO, Fe2O3, V2O5
Answer:
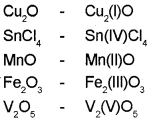
Question 17.
In a redox reaction, oxidation and reduction occur simultaneously.
a) Write the classical concept of oxidation and reduction.
b) Identify the species undergoing oxidation and reduction in the following reaction:
H2S(S) + Cl2(g) → 2HCl(g) + S(s)
Answer:
1. Oxidation:
addition of oxygen/electronegative element to a substance or removal of hydrogen/ electropositive element from a substance.
Reduction:
removal of oxygen/electronegative element from a substance or addition of hydrogen/ electropositive element to a substance.
2.Oxidised species:
H2S. This is because a more electronegative element, Cl is added to H or a more electro positive element, H has been removed from S.
Reduced species:
Cl. This is due to addition of more electropositive element H to it.
Plus One Chemistry Redox Reactions Three Mark Questions and Answers
Question 1.
An equation is given below:
HNO3+ l2 → HlO3 + NO2 + H2O
- Find the oxidising agent and reducing agent.
- Balance the equation using half reaction method.
Answer:
Oxidising agent = HNO3
Reducing agent = l2
Skeletal equation:
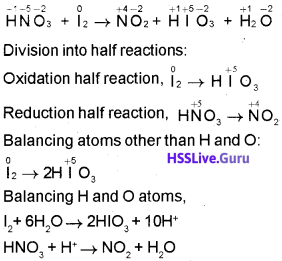
Balancing the charge on the half reactions by adding electrons and equalising the number of electrons:

Question 2.
1. Define redox reactions.
2. Predict whether the following reaction is a redox reaction or not? Justify.
Cr2O72- + H2O → 2CrO42- + 2H+
Answer:
1. Redox reactions are those reactions are those reactions in which reduction and oxidation occur simultaneously. These reactions involve change in oxidation state of the interacting species.
2. No.
Because no element undergoes change in oxidation number.
![]()
Question 3.
a) Find out the oxidising agent and reducing agent in the following reaction:
Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
b) Balance the following redox reaction in acid medium using oxidation number method.
Cr2O72- + Fe2+ → Cr3+ + Fe3+
Answer:
1. Oxidising agent – Ag
ReducingAgent – Cu
2. Assigning oxidation number:

Question 4.
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO4, Cr2O72- and NO3–.
Answer:
H2SO4
(2 × +1) + x+ (4 × -2) = 0
+2 + x – 8 = 0
x – 6 = 0
x = +6
Cr2O72-
2x + 7 ×-2 = -2
2x = -2 + 14
2x = 12
∴ x = +6
NO3–
x + 3 × -2 = -1
x = -1 + 5
x = +4
Question 5.
1. Assign oxidation numbers
(i) P in NaH2PO4
(ii) Mn in KMnO4
(iii) B in NaBH4
(iv) S in H2SO4
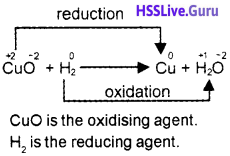
2. Identify the oxidising and reducing agents in the following reaction:
CuO + H2 → Cu + H2O
Answer:
1. i) NaH2PO4
Na+1H2+1PO4-2
1+2 + x- 8 = 0
3 + x – 8 = 0
x – 5 = 0
x =+ 5
ii) K+1MnO4-2
1+ x – 8 = 0
x – 7 = 0
x = +7
iii) Na+1BH4+1
1 + x + 1 × 4 = 0
x + 5 = 0
x = -5
iv) H2+1SO4-2
2 + x – 8 = 0
x – 6 = 0
x = +6
2.
Question 6.
A copper rod is dipped in silver nitrate solution.
- What are the observations?
- Write the displacement reaction.
- Identify the species getting oxidised and reduced.
Answer:
- The colour of the solution changes to blue. Silver is deposited on the copper rod.
- Cu(s) +2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
- Oxidised species – Cu Reduced species – Ag+
![]()
Question 7.
1. Identify the oxidising and reducing agent in the reaction:
CuS + O2 → Cu + SO2
2. Determine the oxidation number of the underlined element in the following:
![]()
Answer:
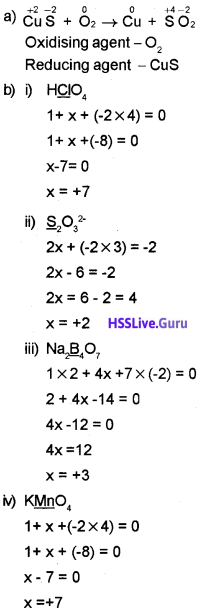
Question 8.
1. Identify the substance oxidised, substance reduced, oxidising agent and reducing agent in the reaction:
Cl2 + 2l– → 2Cl– +l2
2. Calculate the oxidation number of underlined elements in the following compounds:
i) K2\(\underline { Cr } \)2O7
ii) H\(\underline { H } \)O3
Answer:
1. Cl2 is reduced, therefore Cl2 is the oxidising agent. I’ is oxidised, therefore I” is the reducing agent.
2. i) K2\(\underline { Cr } \)2O7
(+1 × 2) + 2x +(-2 × 7) = 0
+2 + 2x – 14 =0
2x – 12 =0
2x = 12
x = +6
ii) H\(\underline { H } \)O3
(+1 × 1) + x + (-2 × 3) = 0
1+ x – 6 = 0
x – 5 = 0
x = +5
Question 9.
Determine oxidation number of the elements underlined in each of the following.

Answer:

Plus One Chemistry Redox Reactions Four Mark Questions and Answers
Question 1.
Permanganate ion (MnO4–) reacts with bromide ion (Br–) in basic medium to give manganese dioxide
(MnO2) and bromate ion (BrO3–).
a) Write the balanced ionic equation for this reaction.
b) Identify the oxidising agent and reducing agent in this reaction.
Answer:

Question 2.
A redox reaction involves oxidation and reduction.
a) What do you understand by electrode potential?
b) Define a redox couple.
c) Explain the set-up for Daniell cell with a diagram.
d) Write the electrode reactions and overall cell reaction which occur in the Daniel cell.
Answer:
a) The potential difference between metal and its own ion is called electrode potential.
b) A redox couple is defined as the combination of oxidised and reduced forms of a substance taking part in an oxidation or reduction half reaction.
c) Take copper sulphate solution in a beaker and put a copper strip. Take zinc sulphate solution in another beaker and put a zinc rod. The two redox couples are represented as Zn2+/Zn and Cu2+/Cu. Put the beaker containing copper sulphate solution and beaker containing zinc sulphate side by side. Connect two solution by a salt bridge. The Zn and Cu rods are connected by a metalic wire with a provision for ammeter and switch. Transfer of electrons now does not take place directly from Zn to Cu2+, but through metallic wire. The electricity from solution in one beaker to solution in the other beaker flows by the migration of ions through the salt bridge.

Question 3.
Redox reactions are those in which oxidation and reduction takes place. Explain the different types of redox reactions with suitable examples.
Answer:
Combination Reactions: The reactions in which two substances combine together to form a new compound are called combination reactions. These can be denoted as A+ B → C where either A or B or both A and B should be in the elemental form.
![]()
Decomposition reactions:
The reactions in which a compound breaks up into two or more substances at least one of which is in elemental form are called decompositions reactions.
![]()
Displacement reactions:
The reactions of the type X + YZ → XZ + Y in which an atom or ion in a compound is displaced by an ion (atom) of another element, such that X and Y are in elemental form are called displacement reactions. They are of two categories:
1. Metal displacement reactions: Reactions in which a more electropositive metal displaces a less electropositive metal from its compound.
![]()
2. Non-metal displacement reactions: These are reactions in which a non-metal is displaced by another metal or non-metal.

Disproportionation reactions: These are special type of redox reactions in which an element in one oxidation state is simultaneously oxidised and reduced. Here one of the reactants should contain an element that should exist in at least three oxidation states. The element in the form of reacting substance is in the intermediate oxidation state; and both higher and lower oxidation states of that element are formed in the reaction.
e.g. The decomposition of hydrogen peroxide.
![]()
Here the oxygen of peroxide, which is present in -1 state, is converted to zero oxidation state in O2 and to -2 state in H2O.
Plus One Chemistry Redox Reactions NCERT Questions and Answers
Question 1.
Fluorine reacts with ice and results in the change :
![]()
Justify that this reaction is a redox reaction.
Answer:
In the given reaction O.N. of F2 changes from zero to -1 in HF and HOF whereas O.N. of oxygen change from -2 in H2O to zero in HOF. Thus, F2 is reduced, whereas oxygen is oxidised and, therefore, it is a redox reaction.
Question 2.
Write formulas for the following compounds:
- Mercury (II) chloride
- Nickel (II) sulphate
- Tin (IV) oxide
- Thallium (I) sulphate
- Iron (III) sulphate
- Chromium (III) oxide
Answer:
- Hg(II)Cl2
- Ni(II)SO4
- Sn(IV)O2
- Tl2(I)SO4
- Fe2(III)(SO4)3
- Cr2(III)O3
![]()
Question 3.
The compound AgF2 is unstable. However, if formed, the compound acts as a very strong oxiding agent. Why?
Answer:
In AgF2, oxidation state of Ag is + 2 which is very unstable. Since Ag can exist in a stable state of + 1 it quickly accepts an electron to form the more stable + 1 oxidation state.
Ag2+ + e– → Ag+
Question 4.
Consider the reactions:
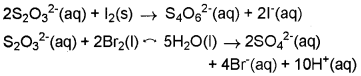
Why does the same reductant, thiosulphate react differently with iodine and bromine?
Answer:
The average O.N. of S in S2O32- is + 2 while in S4O62- it is + 2.5. The O.N. of S in SO42- is+6. Since Br2 is a stronger oxidising agent that l2, it oxidises S of S2O32- to a higher oxidation state of + 6 and hence forms SO42- ion. l2, however, being a weaker oxidising agent oxidises S of S2O32- ion to a lower oxidation of + 2.5 in S4O62- ion.
Question 5.
Why does the following reaction occur?
XeO64-(aq) + 2F–(aq) + 6H+(aq) → XeO3(s) + F2(g) + 3H2O(I)
What conclusion about the compound Na4XeO6 (of which XeO64- is a part) can be drawn from the reaction?
Answer:
The balanced equation along with O.N. of the elements above their symbols will be as:

In the equation the, O.N. of Xe decreases from + 8 in XeO64- to + 6 in XeO3 while that of F increases from – 1 in F– to 0 in F2. Therefore, XeO64- is reduced while F– is oxidised. This reaction occurs because Na4XeO6 (0r XeO64-) is stronger oxidising agent than F2.