You can Download Properties of Matter Questions and Answers, Summary, Activity, Notes, Kerala Syllabus 8th Standard Basic Science Solutions Chapter 4 help you to revise complete Syllabus and score more marks in your examinations.
Kerala State Syllabus 8th Standard Basic Science Solutions Chapter 4 Properties of Matter
Properties of Matter Questions and Answers
Properties of matter
The matter commonly seen in three states, solid, liquid and gas. Matter has volume and mass. Matter is anything which occupies space and has volume. The solid has a definite shape. But liquid occupy the shape of the container. Gas has no definite shape.
Properties Of Matter Class 8 Questions And Answers Question 1.
explain the peculiarities of materials.
Answer:
Tiny particles of matter
The substances are made up of many tiny particles which cannot be seen by naked eye. Even though we cannot see the tiny particles of sugar in sugar solution, we can understand that tiny particles of sugar is dissolved in the solution while we taste it.
All the substances are made up of tiny particles which cannot be seen by naked eye and these tiny particles bear all the properties of the substances.
Properties Of Matter Class 8 Notes Pdf Kerala Syllabus Question 2.
identify and picturise the arrangement of particles in different states of matter.
Answer:
Arrangements of particles in different states of matter
The particles of solids are very close to each other. The freedom of movement of particles is limited. The attractive force between particles is very high.
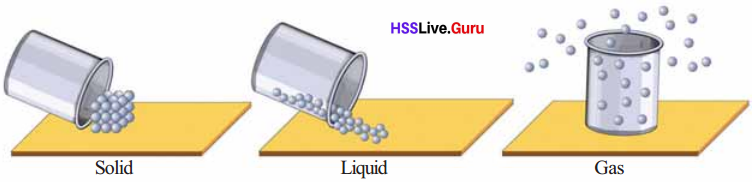
The particles of liquid are relatively further apart and have more freedom for movement than in the solid state. The attractive force is less than that of solid state. The particles in gaseous state remains far away from one another and the attractive force is very low.
Change of State
When ice is heated, it changes into water and when water is further heated it boils and changes into steam.
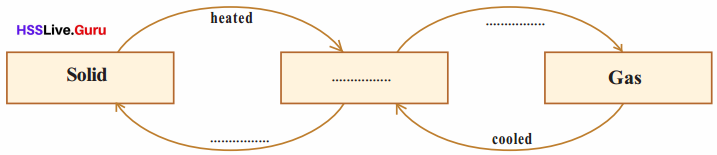
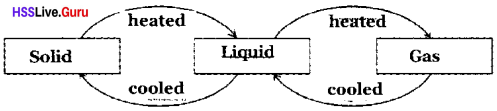
Properties Of Matter Questions And Answers Kerala Syllabus Question 3.
Which form of energy is responsible for the change of state here?
Answer:
Here the form of energy is responsible for the change of state is heat.

When heat is absorbed energy of the particles, distance between the particles, movement of the particles are increased. The attractive force between particles is decreased.
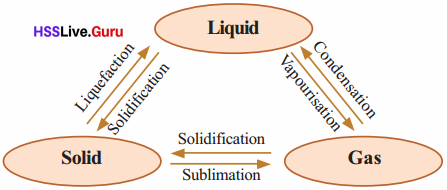
Some substances, when heated, change directly into gas without forming liquid. This phenomenon is sublimation.
Diffusion of substances in different states
The fragrance of incense stick fills the room when the stick is lit. The fragrance spreads because its particles are spread in air. If a drop of ink is added carefully into the water in a beaker, the ink spread in the water.
The spontaneous mixing of different particles having freedom of movement is Diffusion.
Hsslive Guru 8th Class Chemistry Kerala Syllabus Question 4.
Distinguish pure substances and mixtures and tabulate them?
Answer:
Pure Substances and Mixtures
Depending on their nature, substances can be classified into two.
1. Pure Substances
2. Mixtures Materials made of particles of identical nature are called pure substances
Eg: Water, common salt, sugar
The substances made of particles of different nature are called mixtures.
Eg: Salt solution, sugar solution
Separating the Components of a Mixture.
Most of the substances found in nature are mixtures. We use different methods of separation of components in rice mixed with stones, tea mixed with tea dreg, mixture of methanol and ethanol.
Distillation
Distillation is used for separation of components in salt solution.
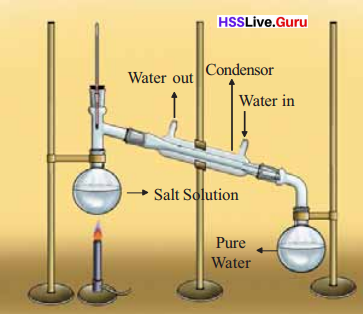
When one component of the mixture is volatile and the others do not vapourise under the same condition, they can be separated by distillation. More over components of a mixture possess a large difference in their boiling points, they can be separated by distillation. Eg:Mixture of water (boiling point ioo°C)and acetone(56°C)
Fractional Distillation
If the boiling points of components have very small differences, fractional distillation is to be used to separate them.
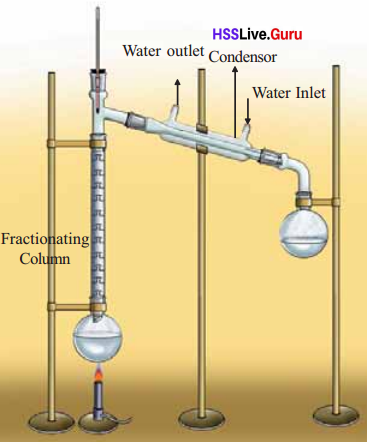
Eg: Mixture of Ethanol (boiling point 78°C) and methanol (boiling point 65°C).
When vapours of the mixture pass through the fractionating column, repeated. liquefaction and vapourisation take place. Subsequently, the vapours of low boiling methanol enter the condenser from the fractionating column, condense to liquid and get collected in the round bottomed flask first. Similarly, ethanol with higher boiling point can be collected later in another round bottomed flask.
Separating Funnel
Separating funnel is an apparatus used for separating immiscible liquids from their mixture.
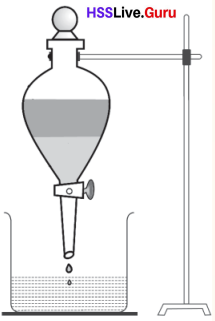
We can use separating funnel to separate the mixture of water and kerosene which are having difference in density.
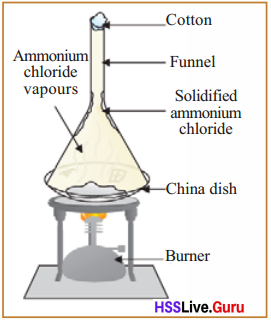
Sublimation
This method can be used to separate the components which have the property of sublimation from the mixture. This method can be used for separating the components of a mixture of ammonium chloride and sand.
Centrifugation
This is a method for separating components from a mixture, based on the difference in the mass of particles. This method is used in clinical laboratories to separate blood cells from blood samples and also for separation of butter from curd.
In order to separate insoluble particles in a liquid mixture on the basis of their mass difference, centrifuge is used. The process is known as centrifugation.
Chromatography
Chromatography is the method used to separate more than one solute dissolved in the same solvent. This method was first employed for separating coloured substances and hence this process came to be known as Chromatography. Chromatography is employed to separate components from dyes and to separate poisonous substances mixed with blood.
Properties of Matter Textbook Questions and Answers
Basic Science Class 8 Solutions Chapter 4 Kerala Syllabus Question 1.
A few mixtures are given below. Tabulate the methods to separate their components and give the reasons for selecting the method.
| Mixture | Method | Reason |
| Common salt and ammonium chloride | ||
| Sugar solution | ||
| Petrol and kerosene | ||
| Camphor and glass powder | ||
| Iron powder and sand |
Answer:
| Mixture | Method | Reason |
| Common salt and ammonium Chlo- | Sublimation | Components having the prope- |
| Sugar solution | Distillation | Components having the property of evaporation |
| Petrol and kerosene | Fractional distillation | Components have small differanee in boiling point |
| Camphor and glass powder | Sublimation | Components having the property of sublimation |
| Iron powder and sand | Magnetic separation | Components having magnetic properties |
8th Class Biology Notes Pdf Kerala Syllabus Question 2.
Given below are certain changes taking place to the particles during change of state. From this, find out and tabulate the changes in the particles when water boils to form steam and also when steam condenses to form water.
- distance increases
- attractive force decreases
- energy increases
- rate of movement increases
- distance decreases
- energy decreases
- attractive force increases
- rate of movement decreases
Answer:
| Water changes to Steam | steam to water |
| Distance increases | Distance decreases |
| Energy increases | Energy decreases |
| Attractive force decreases | Attractive force increases |
| Movement of particles increases | Movement of particles decreases |
Kerala Syllabus 8th Standard Chemistry Notes Question 3.
Spirit kept open in a.watch glass disappears after some time. Which among the following phenomena are responsible for this?
a) sublimation
b) distillation
c) evaporation
d) diffusion
Answer:
a) sublimation
Basic Science For Class 8 Chapter 4 Kerala Syllabus Question 4.
Which are the methods that can be used to separate the components of a mixture made of common salt, ammonium chloride and sand? Write the methods in the order in which they are applied.
Answer:
sublimation → filtration → distillation
Kerala Syllabus 8th Standard Physics Notes Question 5.
Many minerals are present in ordinary water.
a. Which is the method that can used to remove the mineals and obtain pure water?
h.In which type of mixtures is this method employed?
c. Water purified by this method is distilled water .Write two instances of its use.
Answer:
a. distillation
b. When one component of the mixture is volatile and the others do not vapourise under the same condition, they canbe separated by distillation
c. For vaccination, In storage batteries
Basic Science Class 8 Pdf Notes Kerala Syllabus Question 6.
From the following statements, tick (V) those which apply to solid substances alone.
□ Particles have little freedom of movement.
□ Distance between particles is very high
□ Particles remain very close to each other
□ Energy of particles is very high
Answer:
✓ Particles have little freedom of movement.
✓ Particles remain very close to each other
Question 7.
Take a small wooden rectangular block and find its volume (volume = length x breadth x height).
Take a big measuring jar and fill three-fourth of it with water and mark the‘water level. Then dip the block in water in the jar. (To prevent floating, nails can be inserted in the block). Mark the difference in the water level.
a. Is there any relation between the difference in the water level and the volume of the block?
b. Which property of matter is revealed by this experiment?
Answer:
a.Yes. the volume of water rises is the volume of wooden block.
b. The substance that occupies a space.
Question 8.
Electronic balances are very popular now. On an electronic balance, find the weight of an empty balloon. Again, find its weight after filling air. Now, can you find the weight of the air in the balloon?
Repeat the experiment using balloons of different size by filling them with varying quantity of air.
Answer:
Reduce the weight of balloon from the weight of balloon with air
Question 9.
Take water mixed with chalk powder in a bottle. Tie a string to the bottle and swirl it at high speed along a circular path. Observe after sometime.
Repeat the activity using other mixtures which are suspensions. To which method of separation of components of a mixture can this be connected? Are there instances where this principle is made use of. Prepare a note.
Answer:
Centrifugation is the method used. This is a method for separating components from a mixture, based on the difference in the mass of particles. This method is used in clinical laboratories to separate blood cells from blood samples and also for separation of butter from curd.
Question 10.
Take a long white chalk piece and put a mark with black ink slightly above the bottom. Keep the chalk piece dipped perpendicularly in water in a watch glass. After sometime observe the changes. Repeat the experiment using different chalk pieces marked with sketch pens of different colours. To which of the methods of separation you have studied is this related?
Answer:
Chromatography
Properties of Matter Additional Questions and Answers
Question 1.
Find the odd one out
a. Evaporation, Fermentation, Filtration, Chromatography.
b. Sea water, Muddy water, Table salt, Curd, Air
c. Distillation, Separating funnel, Filtration, Evaporation
Answer:
a. Fermentation
b. Table salt
c. Separating funnel
Question 2.
What is the use of chromatography?
Answer:
Solutes dissolved in the same solvent can be separated using chromatography.
Question 3.
What is the importance of evaporation? Where is this technique used on a large scale?
Answer:
Evaporation is used to separate solids dissolved in liquids, mainly water. It is used on a large scale to obtain common salt from sea water. Sea water is left in the open in large containers on sunny days. After a few days salt remains as residue and water evaporates. Common salt is then further purified.
Question 4. What is the advantage of distillation over evaporation?
Answer:
Distillation is more advantageous than evaporation, because liquid can be recover in distillation while in evaporation liquid is lost.
Question 5.
Which process is used to dry clothes in a washing machine?
Answer:
Centrifugation.
Question 6.
Method for separating drugs (medicine) which are miscible in blood Centrifugation, Evaporation, Filtration, Chromatography.
Answer:
Chromatography
Question 7.
From the below statements select correct one?
a. Every substance has a definite mass
b. Every substance has a definite shape
c. Every substance has a definite volume
Answer:
a. Every substance has a definite mass
Question 8.
Melting: Converting solid into liquid Sublimation:
a. Observe the first pair and fill up. the blanks in the second pair suitable.
b. Write two examples of sublimation
Answer:
a. Sublimation. A solid directly changes into gas
b. Camphor, iodine, naphthalene.
Question 9.
From Gopu’s hand the bottle containing coconut oil fall into a bucket containing water. Is it possible to separate oil and water?
Answer:
Using a separating funnel, they can be separated. The denser water will be at the bottom and the lighter oil will be at the top. Water is removed through the tap.
Question 10.
Classify the following substances according to its state and tabulate.
Water, pencil, book, air, kerosene, oxygen
Answer:
| solid | liquid | gas |
| Pencil Book |
Water kerosene |
Air Oxygen |
Question 11.
Will water fill in tumbler when a glass tumbler is immersed perpendicularly into the water in a turf ? Give reason.
Answer:
No because there is air in the tum-bler. Air need space to occupy so water cannot enter into the tumbler.
Question 12.
A balloon filled with air and an empty balloon weigled in a digital balance found difference in weight. What are the properties of matter understood by this experiment?
Answer:
Matter needs space to occupy and has mass.
Question 13.
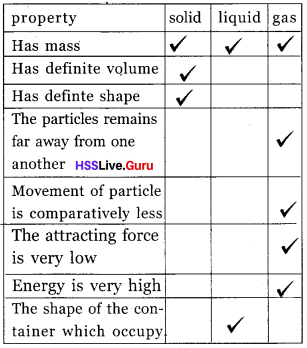
Complete the table.
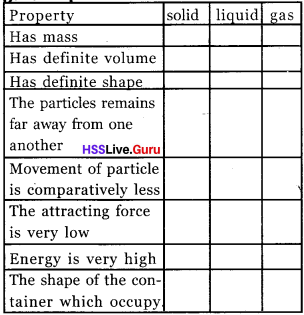
Answer:
Question 14.
Which energy causes the change of state when ice is heated to water and water to water vapour? The three figures shows the arrangements of particles. Recoga- nise and write the state of matter.
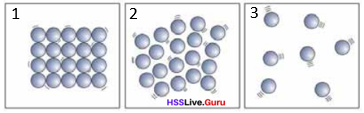
Answer:
Heat energy figure
(1) solid, figure
(2) liquid, figure
(3) gas
Question 15.
Mention three examples from daily life for diffusion.
Answer:
Spreads the smell of perfumes, smell of fruits and food item. Ink spreads in water.
Question 16.
Complete the table
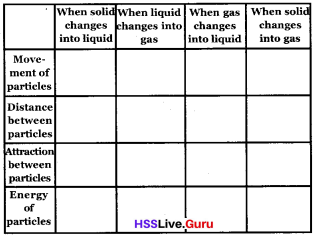
Answer:
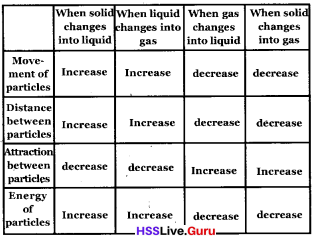
Question 17.
When naphthalene balls are kept somewhere after some days its size diminishes and disappears. Name the phenomenon caused for this process.
Answer:
Sublimation
Question 18.
Mention the reason behind the smell spreads everywhere in the room while in scent stick is lighted. Sublimation, Evaporation, Diffusion
Answer:
Diffusion
Question 19.
Tabulate following as pure substances and mixtures.
Gold, soda water, steam, sugar, soil, water, common salt, sugar solution, carbon dioxide, salt solution.
Answer:
| Pure substances | Mixtures |
| Gold | Soda water |
| Sugar | Sugarsolution |
| Salt | Soil |
| Water | Salt solution |
| Steam | |
| Carbon dioxide |
Question 20.
Find the method of separation of components following mixt¬ures.
a. husk from paddy
b. butter from curd
c. tea dreg from tea
d. salt from sea water
e. blood cells from blood sample
f. mixture of petrol and kerosene
g. salt and ammonia
h. poison from blood
i. water and kerosene
j. water and acetone
Answer:
a. by picking
b. centrifugation
c. filtration
d. distillation
e. centrifugation
f. fractional distillation
g. sublimation
h. chromatography
i. using separating funnel
j. distillation
Question 21.
Write two examples of sublimation from daily life.
Answer:
1.camphor
2. naphthalene balls becomes smaller
Question 22.
Salt, rava and mustard are mixed while Suresh has brought it from shop. Say methods to separate these.
Answer:
Salt dissolves in water. Then filter it. Rava and mustard are separating by picking, salt is collected by distillation.
Question 23.
What will be seen when a filter paper is marked by sketch pen and one end of the paper is dipped in water? what is this process called?
Answer:
different colours are separated. Chromatography
Question 24.
Using given methods write one example for separating components of mixture.
Distillation, magnetic separation, centrifugation, fractional distillation, filtration, sublimation.
Answer:
• Separating common salt from sea water – Distillation
• Mixture if iron powder and sulphur – Magnetic separation
• Butter from curd – Centrifugation
• Separation of kerosene, petrol, die sel – Fractional distillation
• Separating tea dreg from tea – Fil-tration
• Ammonium chloride and common salt – Sublimation
Question 25.
Solid, Liquid, Gas are the three states of matter
a. Which of this having definite volume hut not definite shape?
b. Write any two changes occurs when we change a substance from liquid state to gaseous state?
c. How to convert a gasecfas substances into liquid?
Answer:
a. Liquid
b. Increase the distance between molecules.
• Decrease the attractive force between molecules.
• Increase the speed of molecules.
• Increase the energy of molecules.
c. to be cooled (or) Reduce temperature
Question 26.
Name of the mixtures in column A, properties of components are given in column B, method of separating of component are given in the column C also. Find out the match items from B & C to A.
| Mixture | Character of components | Method of Separation |
| 1. Alcohol and water | difference in boiling point | Centrifugation |
| 2. Soil and mentor | difference in evaporating Character | Distillation |
| 3. Curd and butter | difference in weight of insoluble precipitate | Chromatography |
| Sublimation |
Answer:
| Mixture | Character of components | Method of Separation |
| 1. Alcohol and water | difference in boiling point | Distillation |
| 2. Soil and mentor | difference in evaporating character | Sublimation |
| 3. Curd and butter | difference in weight of insoluble precipitate | Centrifugation |
Question 27.
a. Which device given in the picture?
b. Why should this device doesn’t use for separating petrol and kerosene mixture?
c. Which method is used to separate the mixture containing petrol and kerosene?

Answer:
a. Separating funnel
b. Petrol and kerosene are miscible liquids.
c. Fractional distillation
Question 28.
Diffusion is a phenomenon which
causes the spread of smell.
a. What is the relation between the rate of diffusion and states of substance?
b. What is the relation between diffusion and temperature?
c. Given an example from everyday life which shows relation between diffusion and temperature.
Answer:
a.Rate of diffusion is more in gaseous state, less in solid state.
b. Rate of diffusion increases with increase in temperature.
c. The smell of hot food material spread quickly.
Question 29.
Find out the relation and fill in the blanks.
a. Separate salt from saline water :: Evaporation; Separation of components from black coloured ink :: ………..
b. Distillation :: Condenser; Fractional distillation :: ………..
c. Write down some situations for which the fractional distillation used?
d. When we use fractional distillation for separating components of mixture?
Answer:
a. Chromatography
b. Fractionating column
c. • Separating components to petroleum
• Separating components of air.
d. Used when slight differences in their boiling points.