Students can Download Chapter 14 Environmental Chemistry Notes, Plus One Chemistry Notes helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus One Chemistry Notes Chapter 14 Environmental Chemistry
Introduction
Environmental chemistry deals with various chemical phenomena occurring in the environment. Environmental chemistry deals with the study of the origin, transport, reactions, effects and fates of chemical species in the environment.
Environmental Pollution
The undesirable changes which have harmful effect on plants, animals and human beings is called environmental pollution. A substance, which causes pollution, is known as pollutant.
Atmospheric Pollution
The atmosphere that surrounds the earth is not of ‘ the same thickness at all heights. There are concentric layers of air or regions and each layer has different density. The lowest region of atmosphere in which the human beings along with other organisms live is called troposphere. Above the troposphere, lies stratosphere. Troposphere is a turbulent, dusty zone containing air, much water vapour and clouds. This is the region of strong air movement and cloud formation. The stratosphere, on the other hand, contains dinitrogen, dioxygen, ozone and little water vapour. Atmospheric pollution is generally studied as tropospheric and stratospheric pollution. The presence of ozone in the stratosphere prevents about 99.5 per cent of the sun’s harmful ultraviolet (UV) radiations from reaching the earth’s surface and thereby protecting humans and other animals from its effect.
![]()
Tropospheric Pollution
Tropospheric pollution occurs due to the presence of undesirable solid or gaseous particles in the air. The following are the major gaseous and particulate pollutants present in the troposphere:
1) Gaseous air pollutants:
These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
2) Particulate pollutants:
These are dust, mist, fumes, smoke, smog, etc.
1. Gaseous air pollutants
a) Oxides of Sulphur:
Oxides of sulphur are produced when sulphur containing fossil fuel is burnt. It has been reported that even a low concentration of sulphur dioxide causes respiratory diseases e.g., asthma, bronchitis, emphysema in human beings. Sulphur dioxide causes irritation to the eyes, resulting in tears and redness. High concentration of SO2 leads to stiffness of flower buds which eventually fall off from plants.
2SO2 (g) + O2 (g) → 2SO3 (g)
The reaction can also be promoted by ozone and hydrogen peroxide.
SO2 (g) + O3 (g) → SO3 (g) + O2 (g)
SO2(g) + H2O2(l) → H2SO4(aq)
b) Oxides of Nitrogen :
![]()
NO reacts instantly with oxygen to give NO2
2NO(g) + O2 (g) → 2NO2 (g)
Rate of production of N02 is faster when nitric oxide reacts with ozone in the stratosphere.
NO(g) + O3 (g) → NO2 (g) + O2 (g)
The irritant red haze in the traffic and congested places is due to oxides of nitrogen. Higher concentrations of NO2 damage the leaves of plants and retard the rate of photosynthesis. Nitrogen dioxide is a lung irritant that can lead to an acute respiratory disease in children. It is toxic to living tissues also. Nitrogen dioxide is also harmful to various textile fibres and metals.
c) Hydrocarbons:
Hydrocarbons are composed of hydrogen and carbon only and are formed by incomplete combustion of fuel used in automobiles. Hydrocarbons are carcinogenic, i.e., they cause cancer. They harm plants by causing ageing, breakdown of tissues and shedding of leaves, flowers and twigs.
d) Oxides of Carbon :
i) Carbon monoxide:
Carbon monoxide is mainly released into the air by automobile exhaust. Other sources, which produce CO, involve incomplete combustion of coal, firewood, petrol, etc. Many vehicles are poorly maintained and several have inadequate pollution control equipment resulting in the release of greater amount of carbon monoxide and other polluting gases. CO binds to haemoglobin to form carboxyhaemoglobin, which is about 300 times more stable than the oxygen-haemoglobin complex. In blood, when the concentration of carboxyhaemoglobin reaches about 3-4 per cent, the oxygen carrying capacity of blood is greatly reduced. This oxygen deficiency results into headache, weak eyesight, nervousness and cardiovascular disorder. This is the reason why people are advised not to smoke. In pregnant women who have the habit of smoking the increased CO level in blood may induce premature birth, spontaneous abortions and deformed babies.
![]()
ii) Carbon dioxide:
Carbon dioxide (CO2) is released into the atmosphere by respiration, burning of fossil fuels for energy, and by decomposition of limestone during the manufacture of cement. It is also emitted during volcanic eruptions. Carbon dioxide gas is confined to troposphere only. Normally it forms about 0.03 per cent by volume of the atmosphere. With the increased use of fossil fuels, a large amount of carbon dioxide gets released into the atmosphere. Excess of CO2 in the air is removed by green plants and this maintains an appropriate level of CO2 in the atmosphere. Green plants require CO2 for photosynthesis and they, in turn, emit oxygen, thus maintaining the delicate balance. As you know, deforestation and burning of fossil fuel increases the CO2 level and disturb the balance in the atmosphere. The increased amount of CO2 in the air is mainly responsible for global warming.
Global Warming and Greenhouse Effect
Greenhouse effect:
Though carbon dioxide is not toxic, the excess concentration of it can lead to changes in climatic conditions, especially by raising the global tempreature. Greenhouse ef- feet is the phenomenon in which earth’s atmosphere traps the heat from the sun and prevents it from escaping into outer space resulting in the rise of atmospheric temperature.
The earth’s atmosphere allows most of the sunlight that falls on it to pass through and heats the surface of the earth. But the heat radiated by the heated surface in the form of infrared radiation is absorbed by greenhouse gases such as CO2, CH4, O3, chlorofluoro carbon compound, water vapour etc. Thus these gases prevent the heat radiation of the earth to go out in space.
As more and more infrared, radiations are trapped, the atmosphere becomes hotter and the global temperature rises up. This is known as global warming. There has been a marked increase in the levels of carbon dioxide in the atmosphere due to severe deforestation and burning of fossil
fuels. An increase in average global temperature is likely to increase infectious diseases such as yellow fever, dengue fever etc. Global warming leads to heating up of water which in turn results in an increase of water level in oceans, lakes, etc.
Acid Rain
Rain water has a pH of 5.6 due to the presence of H+ ions formed by the reaction of rainwater with carbon dioxide present in the atmosphere.
H2P(l) + CO2(g) \(\rightleftharpoons \) H2CO3(aq)
H2CO3(aq) \(\rightleftharpoons \) H+(aq) + HCO3–(aq)
![]()
When the pH of the rain water drops below 5.6, it is called acid rain. Acid rain is a byproduct of a variety of human activities that emit the oxides of sulphur and nitrogen in the atmosphere. As mentioned earlier, burning of fossil fuels (which contain sulphur and nitrogenous matter) such as coal and oil in power stations and furnaces or petrol and diesel in motor engines produce sulphur dioxide and nitrogen oxides. SO2 and NO2 after oxidation and reaction with water are major contributors to acid rain because polluted air usually contains particulate matter that catalyse the oxidation.
2SO2(g) + O2 (g) + 2H2O(I) 2H2SO4(aq)
4NO2(g) + O2(g) + 2H2O(I) 4HNO3(aq)
Ammonium salts are also formed and can be seen as an atmospheric haze (aerosol of fine particles). Aerosol particles of oxides or ammonium salts in raindrops result in wet deposition. S02 is also absorbed directly on both solid and liquid ground surfaces and is thus deposited as dry-deposition. Acid rain is harmful for agriculture, trees and plants as it dissolves and washes away nutrients needed for their growth. It causes respiratory ailments in human beings and animals. When acid rain falls and flows as ground water to reach rivers, lakes etc. it affects plants and animal life in aquatic ecosystem. It corrodes water pipes resulting in the leaching of heavy metals such as. iron, lead arid copper into the drinking water. Acid rain damages buildings and other structures made of stone or metal. The Taj Mahal in India has been affected by acid rain.
2. Particulate pollutants
The term particulate refers to finely divided solid or liquid particles suspended in air. The particulates usually present in atmosphere are fly ash, soot, dust, metal particles, asbestos dust, solid hydrocarbons, smoke, sulphuric acid and nitric acid mists.
Minute living organisms such as fungi, moulds, algae etc. dispersed in air are called viable particulates. Particles formed either by breakdown of larger materials or by condensation of minute particles are called non-viable particulates, eg: Mists, fumes, smoke, dust etc.
Smog
The word smog is derived from smoke and fog.
This is the most common example of air pollution that occurs in many cities throughout the world. There are two types of smog:
a) Classical smog
occurs in cool humid climate. It is a mixture of smoke, fog and sulphur dioxide. Chemically it is a reducing mixture and so it is also called as reducing smog.
b) Photochemical smog
occurs in warm, dry and sunny climate. The main components of the photochemical smog result from the action of sunlight on unsaturated hydrocarbons and nitrogen oxides produced by automobiles and factories. Photochemical smog has high concentration of oxidising agents and is, therefore, called as oxidising smog. Formation of photochemical smog – When fossil fuels are burnt, a variety of pollutants are emitted into the earth’s troposphere. Two of the pollutants that are emitted are hydrocarbons (unburnt fuels) and nitric oxide (NO). When these pollutants build up to sufficiently high levels, a chain reaction occurs from their interaction with sunlight in which NO is converted into nitrogen dioxide (NO2). This NO2, in turn, absorbs energy from sunlight and breaks up into nitric oxide and free oxygen atom.
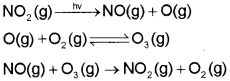
Ozone is a toxic gas and both NO2 and O3 are strong oxidising agents and can react with the unburnt hydrocarbons in the polluted air to produce chemicals such as formaldehyde, acrolein and peroxyacetyl nitrate (PAN).

Effects of photochemical smog
Both ozone and PAN act as powerful eye irritants. Ozone and nitric oxide irritates the nose and throat and their high concentration causes headache, chest pain, dryness of the throat, cough and difficulty in breathing. Photochemical smog leads to cracking of rubber and extensive damage to plant life. It also causes corrosion of metals, stones, building materials, rubber and painted surfaces. Catalytic converters are used in the automobiles, which prevent the release of nitrogen oxide and hydrocarbons to the atmosphere. Certain plants e.g., Pinus, Juniparus, Quercus, Pyrus and Vitis can metabolise nitrogen oxide and therefore, their plantation could help in this matter.
Stratospheric Pollution
Formation and Breakdown of Ozone
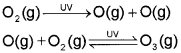
The main reason of ozone layer depletion is believed to be the release of chlorofluorocarbon compounds (CFCs), also known as freons. These compounds are non-reactive, non-flammable, non-toxic organic molecules and therefore used in refrigerators, air conditioners, in the production of plastic foam and by the electronic industry for cleaning computer parts etc. Once CFCs are released in the atmosphere, they mix with the normal atmospheric gases and eventually reach the stratosphere. In stratosphere, they get broken down by powerful UV radiations, releasing chlorine free radical.

![]()
The chlorine radicals are continuously regenerated and cause the breakdown of ozone. Thus, CFCs are transporting agents for continuously generating chlorine radicals into the stratosphere and damaging the ozone layer. Antarctica reported about depletion of ozone layer commonly known as ozone hole over the South Pole. It was found that a unique set of conditions was responsible for the ozone hole. In summer season, nitrogen dioxide and methane react with chlorine monoxide and chlorine atoms forming chlorine sinks, preventing much ozone depletion, whereas in winter, special type of clouds called polar stratospheric clouds are formed over Antarctica. These polar stratospheric clouds provide surface on which chlorine nitrate formed gets hydrolysed to form hypochlorous acid. It also reacts with hydrogen chloride produced as per reaction to give molecular chlorine.

When sunlight returns to the Antarctica in the spring, the sun’s warmth breaks up the clouds and HOCl and Cl2 are photolysed by sunlight, as given in reactions.

Water Pollution
Causes of Water Pollution
(i) Pathogens:
The most serious water pollutants are the disease causing agents called pathogens. Pathogens include bacteria and other organisms that enter water from domestic sewage and animal excreta. Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis which cause gastrointestinal diseases.
![]()
Dissolved oxygen (DO) in water:
The concentration of dissolved oxygen in water is of vital importance to aquatic life. Growth of fish is inhibited if the dissolved oxygen level in waterfalls below 6 ppm. Pollution causes decrease in DO level.
Dissolved oxygen (DO) in water is consumed by micro-organisms to oxidise organic matter in sewage. Deoxygenation of water may also take place by the bio-oxidation of nitrogeneous matter.
Biochemical oxygen demand (BOD) :
It is the amount of dissolved oxygen required by microorganisms to oxidise organic and inorganic matter present in polluted water. It is generally expressed in ppm (parts per million). ‘Clean water1 would have a BOD value less than 5 ppm while highly contaminated water (say, river water) could have a BOD value 17 ppm or more.
![]()
The amount of oxygen in ppm that would be required to oxidise all the contaminants in water is called chemical oxygen demand (COD).
Nowadays most of the detergents available are biodegradable. However, their use can create other problems. The bacteria responsible for degrading biodegradable detergent feed on it and grow rapidly. While growing, they may use up all the oxygen dissolved in water. The lack of oxygen kills all other forms of aquatic life such as fish and plants. Fertilizers contain phosphates as additives. The addition of phosphates in water enhances algae growth. This process in which nutrient enriched water bodies support a dense plant opulation, which kills animal life by depriving it of oxygen and results in subsequent loss of biodiversity is known as Eutrophication.
International Standards for Drinking Water
The International Standards for drinking water are given below and they must be followed.
Fluoride:
For drinking purposes, water should be tested for fluoride ion concentration. Its deficiency in drinking water is harmful to man and causes diseases such as tooth decay etc. Soluble fluoride is often added to drinking water to bring its concentration upto 1 ppm or 1 mg dm-3. The F– ions make the enamel on teeth much harder by converting hydroxyapatite, [3(Ca3(PO4)2.Ca(OH)2], the enamel on the surface of the teeth, into much harder fluorapatite, [3(Ca3(PO4)2.CaF2]. However, F- ion concentration above 2 ppm causes brown mottling of teeth. At the same time, excess fluoride (over 10 ppm) causes harmful effect to bones and teeth, as reported from some parts of Rajasthan.
Lead:
Drinking water gets contaminated with lead when lead pipes are used for transportation of water. The prescribed upper limit concentration of lead in drinking water is about 50 ppb. Lead can damage kidney, liver, reproductive system etc.
Sulphate:
Excessive sulphate (>500 ppm) in drinking water causes laxative effect, otherwise, at moderate levels it is harmless.
Nitrate:
The maximum limit of nitrate in drinking water is 50 ppm. Excess nitrate in drinking water can cause disease such as methemoglobinemia (‘blue baby’ syndrome).
Soil Pollution
Any factor which destroys the quality, texture and mineral content of the soil or which disturbs the biological balance of the organisms in the soil is referred to as soil pollutant.
Soil pollution has adverse effect on plant growth. Soil pollution is mainly due to
- Indiscriminate use of fertilizers, pesticides etc.
- Dumping of waste materials.
- Deforestation.
Large number of pesticides are used to save plants from pests, rats, insects, fungi etc. Chlorinated hydrocarbons, malathion, aldrin, DDT, BHC etc. are some substances used extensively in agriculture. Herbicides are used to kill weeds. Sodium chlorate (NaClO3), sodium arsenite (Na3AsO3) etc. are widely used as herbicides. Inorganic arsenic compounds are toxic to mammals. Fungicides are important because they counter the growth of fungi. Organo-mercury compounds are used to fungicides. However, these compounds break down in the soil causing harmful effects on human beings. Dumping of paper, plastics and other toxic substances in the soil creates serious problems. A large number of heavy metals get deposited in the soil around smelting industries. These effluents, in the long run, pollute the soil.
![]()
Control of environmental pollution
Both industrial and domestic wastes need treatment for safe disposal, (i) Recycling of wasters not only saves the cost on raw materials but also reduces waste disposal costs. Collection and recycling of glass, metal scrap, plastics etc. are some examples of industrial recycling, (ii) Sewage water is filtered to remove large solids and then allowed to settle. Solids can settle as a sludge at the bottom while oil, grease etc. float at the surface which can be skimmed off. The sludge is dried and incinerated at high temperature (above 1000°C) in presence of oxygen. Sewage sludge can be degraded by anaerobic digestion by micro-organisms.
Green Chemistry
Introduction
Green chemistry is a way of thinking and is about utilising the existing knowledge and principles of chemistry and other sciences to reduce the adverse impact on environment. Green chemistry is a production process that would bring about minimum pollution or deterioration to the environment. The byproducts generated during a process, if not used gainfully, add to the environmental pollution. Such processes are not only environmentally unfriendly but ‘ also cost-ineffective. The waste generation and its disposal both are economically unsound. Utilisation of existing knowledge base for reducing the chemical hazards along with the developmental activities is the foundation of green chemistry. It is well-known that organic solvents such as benzene, toluene, carbon tetrachloride etc., are highly toxic. One should be careful while using them.
Green Chemistry In Day-To-Day Life
i) Dry Cleaning of Clothes
Tetra chlroroethene (Cl2C=CCl2) was earlier used as solvent for dry cleaning. The compound contaminates the ground water and is also a suspected carcinogen. Replacement of halogenated solvent by liquid CO2 will result in less harm to ground water. These days hydrogen peroxide (H2O2) is used for the purpose of bleaching clothes in the process of laundry, which gives better results and makes use of lesser amount of water.
ii) Bleaching of Paper
Chlorine gas was used earlier for bleaching paper. These days, hydrogen peroxide (H2O2) with suitable catalyst, which promotes the bleaching action of hydrogen peroxide, is used.
iii) Synthesis of Chemicals
Ethanal (CH3CHO) is now commercially prepared by one step oxidation of ethene in the presence of ionic catalyst in aqueous medium with a yield of 90%.
![]()