Students can Download Chapter 6 General Principle and Processes of Isolation of Elements Notes, Plus Two Chemistry Notes helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus Two Chemistry Notes Chapter 6 General Principle and Processes of Isolation of Elements
Metallurgy – the entire scientific and technological processes used for isolation of the metal from their ores. The extraction and isolation of metals from ores involve the following major steps.
1. Concentration of the ore
2. Isolation/extraction of the metal from its concentrated ore and
3. Purification or refining of the metals
Minerals:
Naturally occuring chemical substance in the earth’s crust obtainable by mining.
![]()
Ores:
minerals from which the metals are economically and profitably extracted. All ores are minerals but all minerals are not ores.
Gangue :
earthly matter or unwanted materials present in ore.
Occurrence of Metals :
Metals are present in earth’s crust as oxides, sulphides, carbonates etc.

Concentration (dressing or benefaction) of Ores:
process of removal of gangue or matrix from the ore. The different process used are:
Hydraulic Washing or Gravity Seperation:
It is based on the differences in gravities. An upward stream of running water is used to wash the powdered ore. The lighter gangue particles are washed away and the heavier ores are left behind.
Magnetic Separation:
It is based on differences in magnetic properties of the ore components. It is carried out if either the ore or the gangue is capable of being attracted by the magnetic field. The powdered ore is carried on a conveyer belt which passes over a magnetic roller.
Froth Floatation Method:
used to separate sulphide ore from the gangue. Here a suspension of the powdered sulphide ore is agitated with collectors and froth stabilisers by passing a forceful current of air. The froth formed which carries the mineral particles is skimmed off and then dried.
‘Depressants’ are used to separate two sulphide ores. e.g. in case of an ore containing ZnS and PbS, the depressant used is NaCN. It selectively prevents ZnS from coming to the froth but allows PbS to come with the froth.
Leaching:
a method of ore concentration by dissolving the ore in a suitable solvent.
a) Leaching of Alumina from Bauxite:
The powdered bauxite ore is digested with concentrated solution of NaOH at 473-523 K and 35 – 36 bar pressure. The Al2O3 is leached out as sodium aluminate leaving the impurities behind. But the impurity SiO2 is also leached out as sodium silicate.
Al2O3 (S)+ 2 NaOH(aq) +3H2O(l) → 2Na[Al(OH)4](aq)
The aluminate solution is neutralised with CO2 gas and hydrated Al2O3 precipitated. At this stage, the slution is seeded with freshly prepared samples of hydrated Al2O3 which induces the precipitation. The sodium silicate remains in the solution.
2Na[Al(OH)4](aq) + CO2(g) → Al2O3 + xH2O(s) + 2 NaHCO3(aq)
The hydrated alumina is filtered, dried and heated to give back pureAl2O3.
![]()
![]()
b) Other example:
In the metallurgy of silver and gold, the ore is treated with a dilute solution of NaCN or KCN. Later the metal ion in the solution is replaced by Zn metal, which acts as the reducing agent.
4M(s) + 8CN–(aq) + 2 H2O(aq) + O2(g) → 4[M(CN)2]–(aq) + 4 OH–(aq) (M = Ag or Au)
2[M(CN)2]–(aq) + Zn(s) [Zn(CN)4]2-(aq) + 2 M(s)
Extraction of Crude Metal from Concentrated Ore:
The concentrated ore must be converted to oxide and then reduced to metal. It involves two steps.
a) Conversion to Oxide
i) Calcination:
process in which the ore is heated strongly in the absence of air.
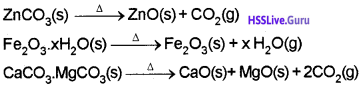
ii) Roasting:
process of heating the ore in a regular supply of air in a furnace at a temperature below the melting point of the metal.
2 ZnS + 3O2 → 2 ZnO + 2SO2
2PbS + 3O2 → 2PbO + 2SO2
2CU2S + 3O2 → 2Cu2O + 2SO2
Flux:
substance which combines with gangue present in the ore and form easily fusible materials called the slag.
Flux + Gangue → Slag (fusible)
FeO + SiO2 → FeSiO3 (Slag)
b) Reduction of Oxide to the Metal:
The metal oxide is reduced by reducing agents (e.g. C, CO or even another metal) which combine with the oxygen of, the metal oxide.
MxOy + yC → xM + yCO
Thermodynamic Principles of Metallurgy:
All those metals which have more negative Gibbs energies of formation of their oxides can reduce the oxides of other metals whose Gibbs energies of formation are less negative.
Ellingham Diagram:
graph of variation of ∆rGΘ vs. T for the formation of metal oxide from metals.

When the value of ∆G is negative in the equation ∆G = ∆H —T∆S then the reaction will proceed. If ∆S is positive, on increasing the temperature(T), the value of T∆S would increase (∆H < T∆S) and then AG will become -ve.
If the reactants and products of the coupled reaction (reduction of the metal oxide and oxidation of the reducing agent) are put together in a system and the net ∆G of the two possible reacfions is -ve, the overall reaction will occur.
Applications of Ellingham Diagram
- It provides a sound basis for considering the choice of reducing agent in the reduction of oxides.
- It helps in predicting the feasibility of thermal reduction of an ore.
Limitations of Ellingham Diagram
- It does not say about the kinetics of the reduction process.
- The interpretations of ∆rGΘ is based on equilibrium constant, K. Thus it is presumed that the reactants and products are in equilibrium. But this is not always true due to changes in entropy values associated with phase transformations.
a) Extraction of Iron from its Oxides:
The concentrated ore is mixed with lime stone and coke and fed into a Blast furnace from its top. Here the oxide is reduced to metal.
FeO(s) + C(s) → Fe(s/ \(\ell \)) + CO(g)
Two simpler reactions such as reduction of FeO and oxidation of coke(C) are are coupled in this process so that the Gibbs energy change of the net reaction is negative.
In Blast furnace, above 710 °C (983 K) coke (C) reduces FeO to Fe. At temperatures below 710 °C (983 K) CO reduces Fe3O4 and Fe2O3to FeO. Hot air is blown from the bottom of the furnace and coke is burnt to give temperature up to 2200 K.
Reactions at lower temperature range (500 – 800 K) –
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2FeO + CO2
Reactions athighertemperature range (900 -1500 K) –
C + CO2 → 2CO
FeO + CO → Fe + CO2
Lime stone is decomposed to CaO which removes silicate impurity of the ore as slag.
CaCO3 → CaO + CO2
CaO + SiO2 → CaSiO3
![]()
Pig iron – iron obtained from the blast furnace which containes about 4% carbon and many impurities.
Cast iron – It contains 3% carbon.
Wrought iron or malleable iron – purest form of commercial iron.
Preparation of Wrought Iron:
It is prepared from; cast iron by oxidising impurities in a reverberatory furnace lined with haematite, which oxidises C to CO.
Fe2O3 + 3C → 2Fe + 3CO
Limestone is added as a flux and S, Si and P are oxidised and passed into the slag. The metal is • recovered and freed from the slag by passing through
rollers.
b) Extraction of Copper:
The sulphide ore (Cu2S) is roasted to give oxide (Cu2O).
2Cu2S + 3O2 → 2Cu2O + 2SO2
The oxide can then be easily reduced to metallic copper using coke. This is because the Cu,Cu2O line is almost at the top in the Ellingham diagram.
Cu2O + C → 2Cu + CO
The ore is heated in a reverberatory furnace after mixing with silica. The iron oxide ‘slags of as iron silicate and copper forms copper matte. This contains Cu2S and FeS. Matte is heated in silica lined converter. The remaining Fe is converted to FeSiO3. The remaining Cu2S and Cu2O undergoes self oxidation-reduction to form blister copper.
2Cu2O + Cu2S → 6 Cu + SO2
The solidified copper obtained has blistered appearance due to the evolution of SO2 and so it is called blister copper.
c. Extraction of Zinc from Zinc Oxide:
ZnO is reduced to metallic Zn by heating with coke.
![]()
The metal is distilled of and collected by rapid chilling.
Electrochemical Principles of Metallurgy:
The metal ions in solution or molten state are reduced by electrolysis or adding some reducing element. For the reduction to be feasible E® should be positive so that ∆GΘ is negative (∆GΘ = – nFEΘ)- During electrolysis, the less reactive metal will come out of the solution and the more reactive metal will go to the solution, e.g.
Cu2+(aq) + Fe(s) → Cu(s) + Fe2+(aq)
More reactive metals have large negative EΘ values. So their reduction is difficult. Sometimes a flux is added for making the molten mass more conducting.
Extraction of Aluminium (Hall-Heroult Process):
Purified Al2O3 is mixed with Na3AlF6 or CaF2 to lower the melting point of the mix and bring conductivity. The fused matrix is electrolysed. Steel cathode and graphite anode are used.

The electrode reactions are:
At cathode : Al3+(melt) + 3e– → Al(l)
At anode : C(s) + O2-(melt) → CO(g) + 2e
C(s) + 2O2- (melt) → CO2(g) + 4e–
Disadvantage:
For each kg of aluminium produced, about 0.5 kg of carbon anode is burnt away as CO and CO2.
The overall reaction is,
2Al2O3 + 3C → 4Al + 3 CO2
Refining:
For obtaining high purity metal, several techniques are used.
a) Distillation:
The impure metal is evaporated to get pure metal, e.g. low boiling metals like Zn, Hg
b) Liquation:
low melting metals like tin and lead are made to flow on sloping surface and thus seperated from high melting impurities.
c) Electrolytic Refining:
Anode – impure metal, Cathode – strip of same metal in pure form, Electrolyte – soluble salt of the same metal. On electrolysis pure metal is deposited at the cathode.
e.g. Electrolytic refining of Cu.
![]()
Anode:
impure Cu, Cathode: pure Cu strip, Electrolyte: acidified solution of CuSO4
During electrolysis Cu in the pure form istransfered from the anode to the cathode. Impurities deposit as anode mud which contains valuable elements like Sb, Se, Te, Ag, Au and Pt. Recovery of these elements meets the cost of refining.
Zn is also refined by electrolytic process.
d) Zone Refining:
This method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal. A circular mobile heater is fixed atone end of a rod of the impure metal. The molten zone moves along with the heater. As the heater moves forward, the pure metal crystallises out of the melt. The process is repeated several times. At one end impurities get concentrated. This end is cut off. e.g., Ge, Si, B, Ga and In.
e) Vapour Phase Refining:
the metal is converted into its volatile compound. It is then decomposed to give pure metal.
Requirements for vapour phase refining:
1. The metal should form a volatile compound with an available reagent.
2. The volatile compound should be easily decomposable, so that the recovery is easy.
i) Mond Process for Refining Nickel:
Nickel is heated in a stream of CO forming a volatile complex, nickel tetracarbonyl.
The nickel tetracarbonyl is heated at high temperature so that it is decomposed to give pure Ni.
ii) van Arkel Method for Refining Zr or Ti:
The crude metal is heated in an evacuated vessel with l2. The metal iodide being more covalent, volatilises.
Zr + 2l2 → Zrl4 (volatile)
The metal iodide is decomposed on a tungsten filament at 1800 K. The pure metal is deposited on the filament.
Zrl4 → Zr + 2l2
Similarly, Ti can be purified.
Ti + 2l2 → Til4 (volatile)
Til4 → Ti + 2 l2
f) Chromatographic Methods:
based on the principle that different components of a mixture are differently adsorbed on an adsorbent.The mixture containing different metal ions are added into the chromatographic column. Different components are adsorbed at different levels on the column. The adsorbed components are removed (eluted) by using suitable solvents (eluant). Column chromatography is very useful for purification of elements which are available in minute quantities, e.g. Inner transition metals are refined by this method.
Uses of Aluminium, Copper, Zinc and Iron
1. Aluminium:
aluminium foils are used as wrappers for chocolates, fine dust of Al is used in paints and lacqures, in the extraction of Cr and Mn from thier oxides, as electricity conductors, for making alloys, e.g. Duralumin (Al + Mg), Alnico (Al + Ni + Co).
2. Copper:
for making wires used in electrical industry, for making water pipes and steam pipes, for making alloys, e.g. brass (Cu + Zn), bronze (Cu + Sn)
3. Zinc:
for galvanising iron, in batteries, as constituent of many alloys, e.g. brass (Cu – 60%, Zn – 40%), german silver (Cu 25-30%, Zn-25-30%, Ni 40 – 50%), zinc dust is used as a reducing agent in the manufacture of dye-stuffs, paints etc.
![]()
4. Iron:
Cast lron:
for casting stoves, railway sleepers, gutter pipes, toys etc; in the manufacture of wrought iron and steel
Wrought Iron:
in making anchors, wires, bolts, chains and agricultural implements.
Steel:
Nickel steel is used for making cables, automobiles and aeroplane parts, pendulum, measuring tapes; Chrome steel is used for cutting tools and crushing machines; Stainless steel is used for cycles, automobiles, utensils, pens etc.