Students can Download Chapter 13 Hydrocarbons Questions and Answers, Plus One Chemistry Chapter Wise Questions and Answers helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 13 Hydrocarbons
Plus One Chemistry Hydrocarbons One Mark Questions and Answers
Question 1.
Which of the following cannot be prepared by Wurtz reaction?
a) CH4
b) C2H6
c) C3H8
d) C4H8
Answer:
a) CH4
Question 2.
The cyclic polymerization of propane produces __________ .
Answer:
1, 3, 5-trimethylbenzene
Question 3.
The reaction
![]()
a) Hydration
b) Dehydration
c) Dehydrogenation
d) Dehalogenation
Answer:
b) Dehydration
Question 4.
Say TRUE or FALSE.
Calcium carbide on hydrolysis gives ethylene.
Answer:
False
![]()
Question 5.
3-Hexyne reacts with Na/liquid NH3 to produce
a) cis-3-Hexene
b) trans-3-Hexene
c) 3-Hexylamine
d) mm2-Hexylamine
Answer:
b) trans-3-Hexene
Question 6.
Fill in the blanks after finding the correct relationship
CH3 – O – CH3 : Ether, CH3-CH2-OH: …………….
Answer:
Alcohol
Question 7.
Choose the correct answer from the brackets given below:
1) General formula of alkene (CnH2n, CnH4n-2)
2) The 2 different forms of elemental carbon (Bitumen, Diamond, Charcoal, Coke, Led, Graphite)
3) Find the odd man out. Give reason (C2H4, C3H6, C4H8, C2H6)
Answer:
1) CnH2n
2) Diamond, graphite
3) C2H6. It is an alkane. All others are alkenes.
Question 8.
The structural formula of a compound and the name given by a student to it is given below:

Answer:
The name given by the student is wrong. The correct name of the compound is 3-Ethyl -2-methyl nonane.
Question 9.
Which is not the isomer of CH3-CH2-O-CH2-CH3? (1)
a) CH3-O-CH2-CH2-CH3
b) CH3-CH2-CH2-CH2-OH
c) CH3-CH2-CO-CH3
d) CH3-CH2(OH)-CH-CH3
Answer:
c) CH3CH2COCH3
Question 10.
Gammexane has the formula
Answer:
C6H6Cl6
Question 11.
Bayer’s reagent is __________ .
Answer:
dil. alkaline KMnO4
Question 12.
The electrophile attacking benzene during nitration is __________ .
Answer:
NO2+
Question 13.
The compound that is least readily nitrated is __________ .
a) phenol
b) Toluene
c) Ethylbenzene
d) Benzoic acid
e) Xylene
Answer:
d) Benzoic acid
![]()
Question 14.
The hydrocarbon formed when Beryllium carbide is treated with water is __________ .
Answer:
Methane
Plus One Chemistry Hydrocarbons Two Mark Questions and Answers
Question 1.
The lUPAC names of 2 compounds with their structural formulae is given below. Make out the errors and correct them.
A. 2, 2-Dimethyl-3-hexyne

B. 1-Butyne
CH3-CH2-CH=CH3
Answer:
A is wrong. The strucutral formula of 2,2-Dimethyl -3- hexyne is
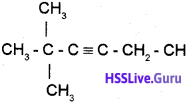
Question 2.
Is there an organic compound named 2-Ethylpentane? Why? If no, write the correct answer.
Answer:
No. When an ethyl group comes with the second carbon atom, the longest chain will have 6 carbon atoms. Hence its correct name will be 3-Methyl hexane.

Question 3.
Write down 2 similarities and 2 difference of the hydrocarbons given below:

Answer:
| Similarities | Dissimilarities |
| Both are unsaturated compounds | Both have different functional group |
| Both hydrocarbons have same word root | One hydrocarbon is alkene and the other is alkyne |
Question 4.
An equation for combined Chemical reaction of acetylene is given below:
![]()
Find the products X’ and ‘Y’?
Answer:

Question 5.
In a Chemistry class, teacher asked students to write the geometrical form of dicarboxylic acid with the formula C4H4O4. For this question, one student wrote
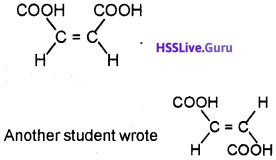
Both of them argued fortheir answers. Hearing this argument teacher told that both of them are right and she also explained the reason for it. Can you write the answer given by the teacher?
Answer:
Two geometrical isomers are possible for dicarboxylic acid. They are cis and transform. In the cis form, similar groups are present on the same side of the double bond whereas in transform, identical groups are present on different side of the double bond.
Question 6.
Match the following:
| Inductive effect | CH3-CH = CH2 |
| Electrometric effect | C6H5-NO2 |
| Hyper conjugation | CH3-CH2 – Br |
| Resonance effect | CH3-CH2(+) |
Answer:
| Inductive effect | CH3 – CH2 (+) |
| Electrometric effect | CH3 – CH2 – Br |
| Hyper conjugation | CH3-CH = CH2 |
| Resonance effect | C6H5-NO2 |
Question 7.
Addition of HBr to propene yields 2-bromopropane, what happens if benzoyl peroxide is added to the above reaction.
Answer:
When propene is allowed to react with HBr in the presence of peroxide, 2 bromo propane is obtained as the minor product and this is called peroxide effect. Anti markonikov’s rule.
Plus One Chemistry Hydrocarbons Three Mark Questions and Answers
Question 1.
Reactions
CH3-C ≡ C-CH3 + H2
Reaction: 2
X + HCl → Y
a) What type of reaction is reaction 1 ?
b) Find X and Y.
c) Which are the different products obtained from the reaction of X with oxygen?
Answer:
a) Addition reaction
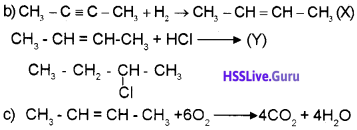
Question 2.
![]()
Here are some functional groups. You are supposed to form 3 structures of hydrocarbons using 3 different functional groups and try to find their names.
Answer:
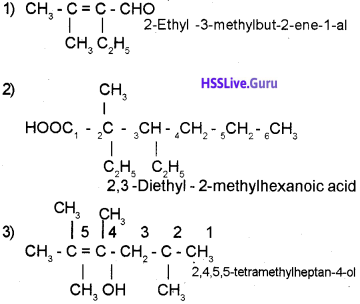
Question 3
CH3 – CH2 – CH2 – CH2 – OH, CH3 – CH2 – CH – CH3
i)For which isomerism can the example above be considered?
ii) Define it.
Answer:
i)Position isomerism
ii) Position isomerism arises as a result of the difference in the position of double bond, triple bond or functional group.
![]()
Question 4.
Explain the following with necessary chemicals equations.
i) Wurtz reaction
ii) Kolbe’s reaction
iii) Ozonolysisof alkenes
Answer:
i) Wurtz reaction – when alkyl halide is allowed to react with metallic sodium in presence of dry ether an alkane is obtained.
![]()
ii) Kolbes reaction – When a solution of sodium acetate is electrolized, ethane is obtained.
![]()
iii) Ozonolysis ofalkene: When an alkene is allowed to react with ozone, an ozonide is obtained. This on hydrolysis gives Aldehyde or ketone. The whole process is called ozonolysis.
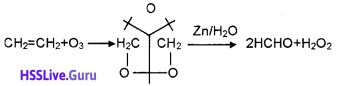
Question 5.
b) Complete the reaction:
Answer:

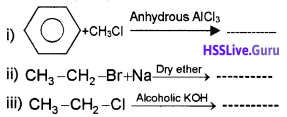
Question 6
1. Explain the reaction between sodium metal and bromoethane in dry ether. (3)
2. Draw Sawhorse and Newman’s projections of the different conformers of ethane.
Answer:
1. 2CH3CH2Br + 2Na → CH3-CH2-CH2-CH3 + 2NaBr
2.
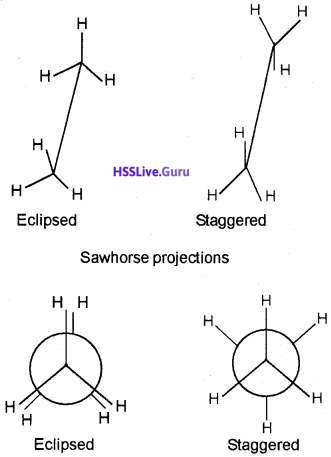
Question 7.
Analyse the given reactions, give the major products. Justify your answer.
a) HBris added to 1-Butene two products are obtained.
b) Action of excess chlorine with benzene in dark.
c) Addition of chlorine to benzene in uv light.
Answer:

c) When Benzene is allowed to react with chlorine in presence of sunlight. Benzene hexa chloride (BHC) is obtained.
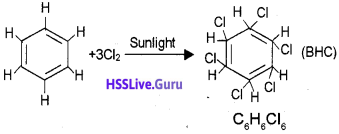
Question 8.
Predict the product in the following reactions and identify the rules:-
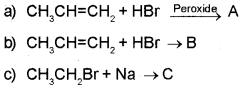
Answer:
a) When propene is allowed to react with HBr in the presence of peroxide, 2-Bromo propane is obtained as the minor product. (Peroxide effect, Kharasch effect or Anti Markownikoffs rule of addition).

Question 9.
Write any two necessary condition for a compound to be aromatic. Convert Acetylene to benzene.
Answer:
Cyclic, Planar and should contain (4n+2)π electrons.

Plus One Chemistry Hydrocarbons Four Mark Questions and Answers
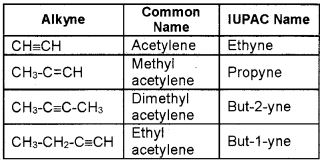
Question 1.
Match the following:

Answer:
Question 2.
Fill in the blanks:

Answer:

Question 3.
Given below are the structures of some hydrocarbons. Pick out the correct IUPAC names for them from the box.
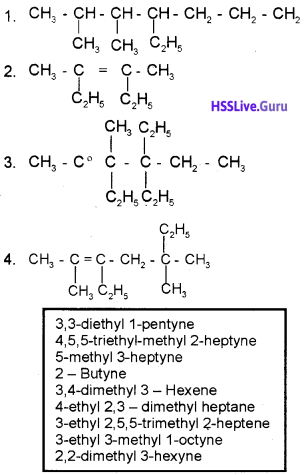
Answer:
1) 4 – Ethyl -2, 3-dimethyl heptane
2) 3, 4-Dimethyl hex – 3-ene
3) 4, 5, 5- triethyl 3 methyl 2 heptyne
4) 3ethyl-2, 5, 5, trimethylheptane
![]()
Question 4.
a) I am an unsaturated hydrocarbon.
b) My wordroot is pent.
c) My suffix is ene. The double bond lies between 2nd and 3rd carbon atoms.
d) l have a branch of methyl group on my second carbon atom. Who am I?
Answer:
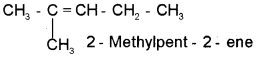
Question 5.
From the given table find out the isomer pairs and which type of isomerism they have?
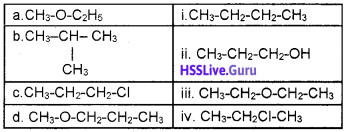
Answer:
Isomer pairs
a – ii – functional group isomerism
b – i – chain isomerism
c-iv-position isomerism
d – iii – metamerism Question 6
Qn 6.
Given below is the structural formula of a compound written by a student.

i) Draw all the possible conformers of the compound?
ii) Arrange them in the order of stability?
Answer:
i)

ii) Eclipsed
Question 7.
A cross word puzzle.
Down
1. Two or more compounds having the same
molecular formula but different physical or chemical properties are known as isomers and the phenomenon is known as
2. Hydrocarbons having the general formula CnH2n.
3. IUPAC name of C6H5CH3.
4. ………….. is added to the word root to show whether the hydrocarbon is saturated or unsaturated.
5. Hydrocarbons contain carbon-carbon triple bond.
Cross
6. From where does the inorganic compounds mainly originate from?
7. Name the alkene with the moelcular formula C10H20
8. Prefix of the functional group carboxylic acid.
9. Compounds with two rings.
10. Compounds having same molecular formula but different arrangements of carbon atoms on either side of the same functional group are called ……..
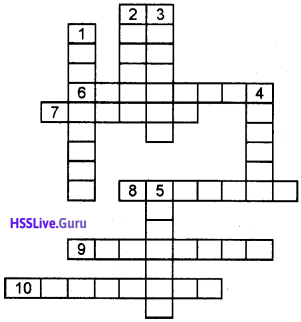
Answer:

Question 8.
1. Name the product obtained when HBr is added to propene. Why?
2. Acetylene is more acidic than ethylene or ethane. Why?
Answer:
1. When propene is treated with HBr, both 2-bromopnopane and 1-bromopropane are formed as products. The major product is2-Bromo-propane. This is due to the rule known as Markonikoffs rule of addition. It states that when a hydrogen halide is added to an unsymmetrical alkene, the halogen atom will goes to the doubly bonded carbon containing lesser number of hydrogen atoms.
2. In Acetylene, the hybridisation of carbon is sp and hence 50% s-character is present.
![]()
Question 9.
a) How will you prepare butane in the laboratory using ethyl bromide (CH3CH2Br) as one of the raw materials. Write relevant equation.
![]()
Identify the product ‘X’. Statethe law that explains the formation of X.
Answer:
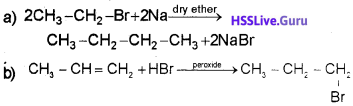
The law of Anti-Markownikoff’s rule of addition explains the formation of 1 -Bromopropane.
Question 10.
1. Addition of HBrto propene yields 2-Bromopropane, while in the presence of Benzoyl peroxide. The same reaction yields 1-Bromopropane. Give reason. Justify your answer.
2. Three compounds are given. Benzene, m- dinitrobenzene and toluene. Identify the compound which will undergo nitration most easily and why?
Answer:
1. When propene is allowed to react with HBr in the presence of “peroxide” 2-Bromopropane is obtained as the minor product. (Peroxide effect or Kharach effect or Anti-Markownikoff’s role of addition) Peroxide effect is applicable only in the case of HBr.

2. Toluene. This is because the -CH3 group, being an activating group activates the benzene ring towards electrophilic substitution in toluene.
Question 11.
a) Write IUPAC names of the products obtained by addition reactions of HBr to hex-1-ene:
i) In the absence of peroxide.
ii) In the presence of peroxide.
b) Howwill you convert:
i) Benzene to toluene
ii) Benzene to nitrobenzene
Answer:
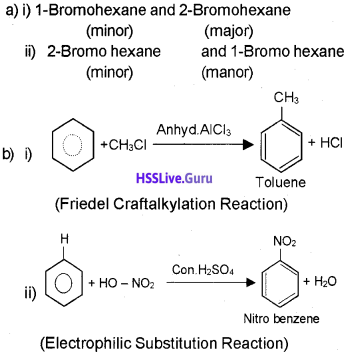
Question 12.
The equations for two chemical reactions are given below:
i) CH ≡ CH + HCl → A → B
ii) OH4 + O2 → C + D
Which are the products A, B, C, and D?
Answer:

Question 13.
The 2 conformations shown below belongs to the compound cyclohexane. Infinite number of conformations are possible for cyclo hexane. But these 2 conformations given below has a peculiarity. Try to find it. Also, define the terms conformers and the phenomenon conformation?
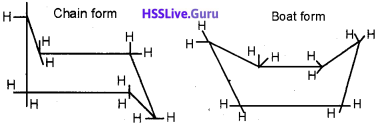
Answer:
Infinite number of conformations are possible for cyclohexane. Out of it chair conformation is the most stable one and the boat conformation is the least stable form.
The different arrangement of a compound which arises as a result of rotation about carbon single bond are called conformers and the phenomenon is called conformation.
Plus One Chemistry Hydrocarbons NCERT Questions and Answers
Question 1.
How do you account for formation of ethane during chlorination of methane? (3)
Answer:
Chlorination of methane takes place through a free radical chain mechanism as given below:

From the above mechanism, it is evident that during chain propagation step, CH3free radicals areproduced. In the chain termination step, the two free CH3 radicals may combine together to form ethane (CH3-CH3) molecule.
![]()
Question 2.
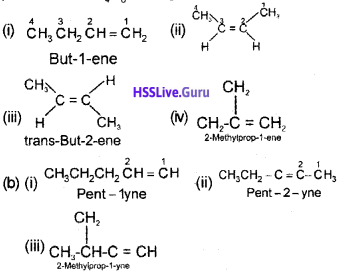
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated: (4)
a) C4H8 (one double bond)
b) C3H8 (one triple bond)
Answer:
a) Isomers of C4H8 having one double bond are:
Question 3.
What are the necessary conditions for any compound to show aromaticaly? (3)
Answer:
The conditions for a compound to show aromaticity are:
i) The molecule must be cyclic
ii) It must have a conjugated system of (4n+2) π- electrones
iii) The molecule must be planar so that delocalization of π-electrones can take place.
Question 4
Explain why the following system are not aromatic?

Answer:
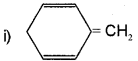
It dose not contain all the π- electrons in the ring. Therefore, it is not an aromatic compound.
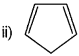
It contains only four electrons, therefore, the system is not aromatic because it dose not contain (4n + 2) π- electrones.
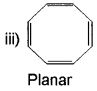
Cyclootatetraene is a conjugated system having 8 π-electrons.
Therefore, the molecule is not contain (4n+2) π-electrons.
Question 5.
In the alkane, H2CCH2C(CH3)2 CH2CH(CH3)2, identify 1°, 2°, 3° carbon atoms and give the total number of atoms bonded to each one of these.
Answer:
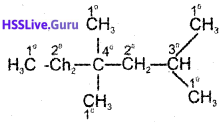
1° Carban atoms = 5
Hydrogen atoms attached to 1° carbon atoms = 15 2° Carbon atoms = 2
Hydrogen atoms attached to 2° carbon atoms = 4 3° Carbon atom = 1
Hydrogen atoms attached to 3° carbon atom = 1
![]()
Question 6.
What effect does branching of an alkane chain has on its melting point?
Answer:
As the branching increases melting point increases.
Question 7.
Why does benzene undergo electrophilic subsitution easily and nucleophilic substitutions with difficulty? (2)
Answer:
Benzene molecule has two n cloud rings, one above and the other below the plane of atoms. Therefore, it is likely to be attached by electrophiles which subsequently brings about substitution.
The nucleophiles would be repelled by the π-electron rings and hence benzene reacts with nucleophiles with difficulty.