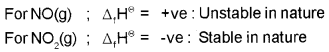Students can Download Chapter 6 Thermodynamics Questions and Answers, Plus One Chemistry Chapter Wise Questions and Answers helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 6 Thermodynamics
Plus One Chemistry Thermodynamics One Mark Questions and Answers
Question 1.
Hot coffee in a thermos flask is an example of system.
Answer:
Isolated
Question 2.
Which of the following statements is incorrect about internal energy?
a) The absolute value of internal energy cannot be determined
b) The internal energy of one mole of a substance is same at any temperature or pressure
c) The measurement of heat change during a reaction by bomb calorimeter is equal to the internal energy change
d) Internal energy is an extensive property
Answer:
b) The internal energy of one mole of a substance is same at any temperature or pressure
Question 3.
For which of the following the standard enthalpy is not zero?
a) C (Diamond)
b) C (Graphite)
c) Liquid mercury
d) Rhombicsulphur
Answer:
a) C (Diamond)
Question 4.
Say TRUE or FALSE?
Any spontaneous process must lead to a net increase in entropy of the universe.
Answer:
TRUE
Question 5.
The ∆H fora reaction is-30 kJ. On the basis of this fact, we can conclude that the reaction
a) Gives off thermal energy
b) Is fast
c) Is slow
d) Is spontaneous
Answer:
a) Gives off thermal energy
![]()
Question 6.
Write the type of system in each of the following:
- Hot water taken in an open vessel
- Hot water taken in a closed metallic vessel
- Hot water taken in a thermos flask
Answer:
- Open system
- Closed system
- Isolated system
Question 7.
In a reversible process the total change in entropy is ∆s(universe) is
Answer:
Zero
Question 8.
For the reaction Ag2O \(\rightleftharpoons \) 2Ag + \(\frac{1}{2}\)O2(g) ∆S and ∆H are 66J K-1mol-1 and 30.56 Kg mol respectively. The reaction will not be spontaneous at.
Answer:
463K
Question 9.
One mole of methane undergoes combustion to form CO2 and water at 25°C. The difference between ∆U & ∆H will be
Answer:
-2RT
![]()
Question 10.
A gas expands from 1 l to 6 l against a constant pressure of 1 atm and it absorbs 500J of heat ∆μ is
Answer:
-6.5J
Question 11.
Born Haber cycle is to find out __________
Answer:
lattice energy
Plus One Chemistry Thermodynamics Two Mark Questions and Answers
Question 1.
1. Explain enthalpy of fusion.
2. Give illustration of fusion of ice.
Answer:
1. It is the enthalpy change when one mole of a solid is converted into its liquid at its melting point.
2. Enthalpy of fusion of ice is 6 kJ/mol. From this it is clear that 6 kJ of energy is required to convert one mole of ice (18 g) into water at 0°C.
Question 2.
a) What do you meant by enthalpy of vapourisation?
b) Explain enthalpy of sublimation.
Answer:
a) It is the enthalpy change when one mole of a liquid is converted into its vapour at its boiling point.
b) It is the enthalpy change when one mole of a solid is converted into its vapour at its transition temperature.
![]()
Question 3.
One equivalent of an acid reacts completely with one equivalent of a base in dilute solution.
1. Which type reaction is this?
2. HCl + NaOH → NaCl + H2O
On the basis of above equation, explain enthalpy of neutralisation.
Answer:
1. Nneutralisation.
2. When one equivalent of HCl (36.5 g) reacts completely with one equivalent of NaOH (40 g), 57.1 kJ energy is liberated.
Question 4.
1. What is the difference between system and surroundings?
2. There are different types of systems. What are they? Explain.
3. Give example for different types of systems.
Answer:
1. A system in thermodynamics refers to that part of universe in which observations are made. The remaining part of the universe other than the system constitutes the surroundings.
2. System is classified into the following three types. Open system: This is a system in which there is exchange of energy and matter between system and surroundings.
Closed system:
This is a system in which there is no exchange of matter, but exchange of energy is possible between system and the surroundings.
Isolated system:
This is a system in which there is no exchange of energy or matter between the • system and the surroundings.
3. Open system
Presence of reactants in an open beaker
Closed system
Presence of reactants in a closed vessel made of conducting material
Isolated system
Presence of reactants in a • thermos flask or any other closed insulated vessel
![]()
Question 5.
Match the following:
| A | B |
| 1. Isothermal | Temperature varies |
| 2. Adiabatic | Temperature constant |
| 3. Isobaric | Volume constant |
| 4. Isochoric | Pressure constant |
Answer:
| A | B |
| 1. Isothermal | Temperature constant |
| 2.Adiabatic | Temperature varies |
| 3. Isobaric | Pressure constant |
| 4. Isochoric | Volume constant |
Question 6.
1. What is meant by enthalpy?
2. Derive an equation for enthalpy change.
3. What is enthalpy change?
Answer:
1. Enthalpy is the sum of internal energy and pressure volume energy.
i.e.,H = U + pV
2. ∆H = ∆U + ∆pV)
∆H = ∆U + p∆V + V∆p
At constant pressure, ∆p=0
∆H = ∆U+p∆V
But ∆U=q+w
∆H=q+w+p∆V
w = -p∆V
i.e; ∆H= q – p∆V + p∆V
∆H = qp
3. Enthalpy change is heat absorbed or released at constant pressure.
Question 7.
1. Find the enthalpy of the reaction,
C(graphite) + O2(g) → CO2(g)
Given,
i) C(graphite)+ ½ O2(g) → CO(g); ∆H =-110.5 kJ mol-1
ii) CO(g) + ½ O2(g) → CO2(g); ∆H =-283.0 kJ mol-1
2. Melting of ice is a spontaneous process. What are the criteria for spontaneity of a process?
Answer:
1. Considerthe reaction,
C(grahite) + O2(g) → CO2 (g); ∆H = x
CO2 can also be prepared through the following two steps:
i) C(graphite)+ ½ O2(g) → CO(g); ∆H =110.5 kJ mol-1
ii) CO(g) + ½ O2(g) → CO2(g); ∆H =-283.0 kJ mol-1
Then by Hess’s law, x = (-110.5+-283.0) kJ=-393.5 kJ
2. Certain endothermic process are found to be spontaneous in nature. Hence, spontaneous behaviour of a process cannot be explained only on the basis of energy consideration.
For a spontaneous process ∆STotal is +ve.
For a nonspontaneous process ∆STotal is -ve.
Question 8.
Explain the following:
- Enthalpy of atomization
- Enthalpy of solution at infinite dilution
Answer:
- It is the enthalpy change on breaking one mole of bonds completely to obtain atoms in the gas phase.
- It is the enthalpy change observed on dissolving the substance in an infinite amount of solvent when the interactions between the ions or solute molecules are negligible.
Question 9.
The enthalpy change for the reaction,
N2(g) + 3H2(g) → 2NH3(g) is -92.38 kJ at 298 K.
What is ∆U at 298 K?
Answer:
∆U = ∆H — ∆ngRT
= -92.38 × 10³ J – [-2 × 8.314J K-1 mol-1 × 293 K)]
= -92.38 × 10³J +4.872 × 10³J
= -87.51 × 10³J
= – 87.51 kJ
![]()
Question 10.
What are the two types of heat capacities? How they are related?
Answer:
The two types of heat capacities are heat capacity at constant pressure (Cp) and heat capacity at constant volume (Cv). These two are related as Cp – Cv = R, where R is the universal gas constant.
Question 11.
Enthalpy and Entropy changes of two reactions are given below: Find out whether they are spontaneous or not at 27°C. Justify.
1. ∆H = 26 kJ/mole, ∆S = 8.3 J/K/mole
2. ∆H = -393.4 kJ/mole, ∆S = 6 J/K/mole
Answer:
1. ∆G = ∆H -T∆S
= 26000 – 300 × 8.3 = 23.510
Since ∆G is positive, the process is non-spontaneous.
2. ∆G = ∆H -T∆S
= -393400 – 300 × 6 = -391600
Since ∆G is negative, the process is spontaneous.
Question 12.
1. What is enthalpy of solution?
2. What is enthalpy of dilution?
Answer:
1. Enthalpy of solution is the enthalpy change associated with the addition of a specified amount of solute to the specified amount of solvent at a constant temperature and pressure.
2. Enthalpy of dilution is the heat withdrawn from the surroundings when additional solvent is added to the solution. It is dependent on the original concentration of the solution and the amount of solvent added.
Question 13.
What is the significance of the second law of thermodynamics in the spontaneity of exothermic and endothermic reactions?
Answer:
The second law of thermodynamics provides explanation for the spontaneity of chemical reactions. In exothermic reactions heat released by the reaction increases the disorder of the surroundings and overall 1 entropy change is positive which makes the reaction spontaneous.
In the case of endothermic reactions, since heat is ‘ absorbed by the system from the surroundings, the entropy change of the surroundings becomes negative (∆Ssurr < 0). In this case the process will be spontaneous only if the entropy change of the reacting system is postive (∆Ssys > 0) and is also greater than ASsurr in magnitude so that the overall entropy change (∆Stotal) is positive.
Question 14.
Explain the importance of third law of thermodynamics.
Answer:
The importance of third law of thermodynamics lies in the fact that it permits the calculation of absolute values of entropy of pure substances from thermal data alone. For a pure substance, this can be done by summing \(\frac { { q }_{ rev } }{ T } \) increments from 0 K to 298 K.
![]()
Question 15.
C12H22O11 + 12O2 → 12CO2 + 11H2O
Consider this equation and answer the following questions.
a) Thermodynamically, which type reaction is this?
b) What is enthalpy of combustion?
c) Give another example.
Answer:
a) Combustion.
b) It is the enthalpy change when one mole of a substance undergoes complete combustion in excess of air or oxygen.
c) C6H12O6 + 6O2 → 6CO2 + 6H2O
![]()
Question 16.
Bond dissociation energies of hydrogen and nitrogen are 430 kJ and 41.8 kJ respectively and the enthalpy of formation of NH3 is – 46 kJ. What is the bond energy of N-Hbond?
Answer:
3H2 + N2 → 2NH3; AH = -46 kJ
3 × ½H2 + ½N2 → 2 × ½NH3
[(3× ½ × 430) + (½ × 941 .8)] – (3N-H) = – 46
[(3 × 215) + (470.9) + (46)] → [3N-H]
[645 + 470.9 + 46] = 3N-H
N-H = 387.3 kJ
Plus One Chemistry Thermodynamics Three Mark Questions and Answers
Question 1.
In 1840, G.H.Hess (a Russian chemist) proposed an important generalisation of thermochemistry which is known after his name as Hess’s law.
1. State Hess’s law.
2. Give illustration of Hess’s law.
Answer:
1. Enthalpy change in a chemical reaction is same whether it takes place in one step or in more than one step.
2. Considerthe formation of CO2.
C + O2 → CO2; ∆H = x
CO2 can be prepared through the following two steps:
C + ½O2 → CO ; ∆H = y
CO + ½O2 → CO2; ∆H = Z
Then by Hess’s law,
x = y + z
Question 2.
∆U = q-p∆V. If the process is carried out at constant
volume, then ∆V=0. Answer the following questions.
1. Give the equation for ∆U.
2. 1000J was supplied to a system at constant volume. It resulted in the increase of temperature of the system from 45 °C to 50 °C. Calculate the change in internal energy.
Answer:
1. ∆U = qv
2. Since the volume kept constant, ∆V=0
∴ ∆U = qv = 1000J
Question 3.
Thermodynamics deals with macroscopic properties.
1. What is the difference between extensive and intensive properties?
2. Classify the following properties into extensive and intensive.
Pressure, Mass, Volume, Temperature, Density, Heat capacity, Viscosity, Surface tension, Internal • energy, Molar heat capacity, Refractive index, Enthalpy, Specific heat capacity
Answer:
1. Extensive properties are those properties whose value depend on the quantity or size of matter present in the system.
Intensive properties are those properties which do not dependent on the quantity or size of matter present in the system.
2. Extensive properties: Mass, Volume, Heat capacity, Internal energy, Enthalpy Intensive: Pressure, Temperature, Density, Viscosity, Surface tension, Molar heat capacity, Refractive index, Specific heat capacity.
![]()
Question 4.
1. What is meant by state of the system and state variables?
2. Give any four examples for state variables/state functions.
Answer:
1. The state of a system refers to the conditions of existence of a system when its macroscopic properties have definite values. If any of the macroscopic properties of the system changes, the state of the system will change. The measurable properties required to describe the state of a system are called state variables or state functions. A state function is a property of a system whose value depends only upon the initial and final states of the system and is independent of the path by which this state has been reached. Properties whose values depend on the path followed are called path functions.
2. State variables/State functions – Temperature, Pressure, Enthalpy, Entropy
Question 5.
1. Explain the Zeroth law of thermodynamics.
2. What are the important modes of transference of energy. Explain.
Answer:
1. The Zeroth law of thermodynamics states that if two bodies say, ‘A’ and ‘B’ are in thermal equilibrium with another body say, ‘C’, then the bodies A’ and ‘B’will also be in thermal equilibrium with each other. It provides the basis for the measurement of temperature.
2. The two important modes of transference of energy are heat and work.
Heat:
The exchange of energy, which is a result of temperature difference between system and surroundings is called heat (q).
Work:
The exchange of energy between system and surroundings can occur in the form of work which can be mechanical work, electrical work or pressure-volume work. The exchange of energy as pressure-volume work can occur if system consists of gaseous substance and there is a difference of pressure between system and surroundings.
Question 6.
1. Explain the symbols and sign conventions of heat and work.
2. Explain internal energy.
Answer:
1. Heat is represented by the symbol ‘q’. The ‘q’ is positive, when heat is transferred from the surroundings to the system and ‘q’ is negative when heat is transferred from system to the surroundings.
Work is represented by the symbol ‘w’. The ‘w’ is positive when work is done on the system and ‘w’ is negative when work is done by the system.
2. Every substance is associated with a definite amount of energy due to its physical and chemical constitution. This is called internal energy. It is the sum of the different types of energies such as chemical, electrical, mechanical etc.
Question 7.
Fill in the blanks.
- If heat is released, ‘q’ is ……………
- For exothermic process ‘∆H’is ………………
- If work is done on the system, ‘w’ is ………………
- For endothermic process ‘∆H’ is ………………
- If work is done by the system, ‘w’ is ………………
Answer:
- Negative
- Negative
- Positive
- Positive
- Negative
Question 8.
1. What is meant by enthalpy of formation?
2. What is the value of standard enthalpy of formation (∆fH) of an element?
Answer:
1. Enthalpy of formation is the enthalpy change when one mole of a compound is formed from its elements in their most stable states of aggregation (i.e., reference state).
2. Zeno
Question 9.
First Law of thermodynamics is the law of conservation of energy.
- Give the mathematical form of the first law.
- Write the Gibb’s equation.
- What is the sign for ∆G for a spontaneous process?
Answer:
- ∆U=q+w w = work done
q = heat absorbed - G = H- TS or ∆G= ∆H – T∆S
- In the case of spontaneous process ∆G = -ve
![]()
Question 10.
1. Predict the sign of ∆S for the reaction,
NH3(g) + HCl(g) → NH4Cl(s)
2. The reaction between gaseous hydrogen and chlorine is
H2(g) + Cl2(g) → 2HCl(g); ∆rH = -1840 kJ
i) What is the enthalpy of formation of HCl?
ii) How much heat will be liberated at 298 K and 1 atm for the formation of 365 g of HCl?
Answer:
1. ∆S is negative
2. i) ∆fH = \(\frac{-1840}{2}\) = -920 kJ mol-1
ii) Heat liberated during the formation of 1 mole (36.5 g) of HCl = 920 kJ
∴ Heat liberated during the formation of 365 g of HCl = 9200 kJ
Question 11.
Derive the Meyer’s relationship.
Answer:
We have, q = C × ∆T
At constant volume, qv = Cv × ∆T = ∆U
At constant pressure, qp = Cp × ∆T = ∆H
For 1 mole of an ideal gas,
∆H = ∆U + ∆(pV) = ∆U + ∆(RT) = ∆U + R∆T
∴ ∆H = ∆U + R∆T
On putting the values of ∆H and ∆U,
Cp∆T = Cv∆T + R∆T
Cp = Cv + R
Cp – Cv = R, which is the Meyer’s relationship.
Question 12.
1. In a process 701J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy for the process?
2. What is free expansion? What is the work done during free expansion of an ideal gas?
Answer:
1. ∆U=q+w = 9 + w = 701J – 394 J = 307J
2. Expansion of a gas in vacuum is called free expansion. No work is done during free expansion of an ideal gas whether the process is reversible or irreversible.
Question 13.
1. Name the instrument used for measuring the ∆U of a process.
2. What is the value of ∆G for a reaction at equilibrium?
3. ∆H and ∆S of a reaction are 30.56 and 0.666 kJ/ mol respectively at 1 atm pressure. Calculate the temperature at which the reaction is in equilibrium.
Answer:
1. Bomb calorimeter
2. Zeno
3. ∆H-T∆S = 0 or ∆H = T∆S
![]()
Question 14.
Thermodynamic process differ based on the manner
in which it is carried out.
1. Distinguish between reversible and irreversible processes.
2. Calculate the amount of work done when 2 moles of a gas expands from a volume of 2 L to 6 L isothermally and irreversibly against a constant external pressure of 1 atm.
Answer:
1.
| Reversible process | Irreversible process |
| 1) Which can be reversed | 1) Which cannot be reversed spontaneous process |
| 2) Takes place infinitesimally slowly | 2) Takes place spontaneous |
| 3) Work done maximum | 3) Work done minimum |
2. w = -p∆V = -1 × (6 – 2)
= – 4 L atm
![]()
Question 15.
1. What are thermochemical equations?
2. Give an example for a thermochemical equation.
Answer:
1. A balanced chem ical equation together with the value of its ∆rH is called a thermochemical equation.
2.
Question 16.
1. Define lattice enthalpy of an ionic compound.
2. What is Born-Haber cycle?
Answer:
1. The lattice enthalpy of an ionic compound is the enthalpy change which occurs when one mole of an ionic compound dissociates into its ions in gaseous state.
2. It is a simplified method developed by Max Born and Fritz Haberto correlate lattice enthanpies of ionic compounds to otherthermodynamic data.
Question 17.
Predict what happens to entropy in the following changes:
- Metal is converted into alloy.
- Solute crystallizes from solution.
- Hydrogen molecule dissociates.
Answer:
- The entropy will increase.
- The entropy will decrease.
- The entropy will increase.
Question 18.
1. Give the relation between change in enthalpy and change in free energy.
2. Name the above relation.
3. What is the significance of the above relation?
Answer:
1. ∆G = ∆H – T∆S
2. Gibbs equation orGibbs-Helmholtz equation.
3. This relation is used to predict the spontaneity of a process based on the value of ∆G . If ∆G is negatve, the process is spontaneous. If ∆G is positive, the process is non-spontaneous.
![]()
Question 19.
1. Predict in each of the following whether entropy increases or decreases.
i) Sublimation of camphor
ii) 4Fe(s) + 3O2(g) → 2Fe2O3(g)
2. The equilibrium constant for a reaction at 30 °C
2.5 x 10-29. What will be the value of ∆G?
Answer:
1. i) entropy increases
ii) entropy increases
2.
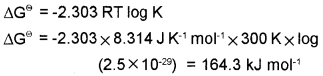
Question 20.
1. Explain the effect of temperature on the spontaneity of a process based on Gibbs equation.
2. For a reaction 2A(g) + B(g) → 2D(g), enthalpy and entropy changes are – 20.5 kJ mol-1 and – 50.4 J K-1mol-1 respectively. Predict whether the reaction occurs at 25 °C.
Answer:
1. If ∆H is -ve and ∆S is +ve, ∆G would certainly be -ve and the process will be spontaneous at all temperatures.
If both ∆H and ∆S are – ve ∆G would be -ve if ∆H > T∆S
If both ∆H and ∆S are + ve ∆G would be -ve if T∆S > ∆H
If ∆H is +ve and AS is -ve, ∆G would certainly be +ve and the process will be non-spontaneous at all temperatures.
2. According to Gibbs equation, ∆G = ∆H – T∆S
∆G = (-20.5 × 10³)-(298 ×-50.4)
= – 20500 + 15019.2 = – 5480.8 J mol-1
Since ∆G is -ve, the process is spontaneous.
Plus One Chemistry Thermodynamics Four Mark Questions and Answers
Question 1.
1. Explain the first, second and third laws of thermodynamics.
2. What do you meant by entropy?
3. Explain the spontaneous process.
Answer:
1. First law:
Energy can neither be created nor be destroyed. Energy in one form can be converted into another form without any loss or gain.
Second law:
Entropy of the universe increases during a spontaneous process.
Third law:
Entropy of a perfect crystalline substance is zero at absolute zero of temperature.
2. Entropy:
Entropy is a measure of randomness or disorder of a system.
3. Spontaneous process:
A spontaneous process is defined as an irreversible process which has a natural tendency to occur either of its own or after proper initiation under the given set of conditions.
![]()
Question 2.
U1, q, w, U2 are given. U1 is internal energy, q is absorbed heat, w is work done and U2 is final energy.
a) Derive an equation for ∆U.
b) Give the equation for w.
c) Calculate the change in internal energy of a system which absorbs 200 J of heat and 315 J of work is done by the system.
Answer:
a) U2 = U1 + q +w
U2 – U1 = q + w
or ∆U = q + w
b) w= -p∆V
c) q = 200J
w = -315J
∆U = ?
∆U = q + w
= 200 + {315}
= 200-315 = -115J
Question 3.
a) Predict whether entropy increases or decreases in the following changes:
i) l2(s) → l2(g)
ii) Temperature of a crystalline solid is raised from 0 Kand 115 K.
iii) Freezing of water
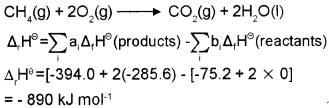
b) Calculate the enthalpy of combustion of methane. Given that standard enthalpies of formation of CH4, CO2 and H2O are -75.2, -394 and -285.6 kJ/mol respectively.
Answer:
a) i) Entropy increases
ii) Entropy increases
iii) Entropy decreases
b) The required equation is,
Plus One Chemistry Thermodynamics NCERT Questions and Answers
Question 1.
In a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy for the process? (2)
Answer:
Heat absorbed by the system, (q) = + 701 J
Work done by the system (w) = – 304 J
Change in internal energy (∆U) = q + w
= 701 – 394
= 307 J
Question 2.
The reaction of cyanamide, NH2CN (s) with oxygen was carried out in a bomb calorimeter and AU was found to be – 742.7 kJ mol1 at 298 K. Calculate the enthalpy change for the reaction at 298 K. (2)
Answer:
NH2CN(S) + 3/2O2(g) → N2(g) + CO2(g) + H2O(l)
∆U = 742.7 kJ mol-1;
∆n(g) =2 – 3/2= + 0.5
R = 8.314 × 10-3kJ K-1 mol-1;
T = 298K
According to the relation, ∆H = ∆U + ∆ngRT
∆H = -742.7 kJ + 0.5 mol × 8.314 × 10-3kJ K-1 mol-1 × 298 K
=-742.7 kJ + 1.239kJ
=-741.5 kJ
![]()
Question 3.
Calculate the number of kJ of heat necessary to raise the temperature of 60 g of aluminium from 35 °C to 55 °C. Molar heat capacity of Al is 24 J mol-1 K-1. (2)
Answer:
Moles of Al (n) = \(\frac { 60{ g } }{ 27{ g }{ mol }^{ -1 } } \) = 2.22 mol
Molar heat capacity (Cm) = 24 J mol-1 K-1
∆T = 55 °C – 35 C° = 20C° or 20 K
Now, q = Cm × n × ∆T
= 24.0 J mol-1 K-1 × 2.22 mol × 20 K
= 1065.6 J
= 1.067 kJ
Question 4.
The enthalpy of formation of CO(g), CO2 (g), N2O (g), N2O4 (g) are -110, – 393, 81 and 9.7 kJ mol-1 respectively. Find the value of ∆rH for the reaction:
N2O4 (g) + 3CO(g) → N2O(g) + 3CO2(g)
Answer:

Question 5.
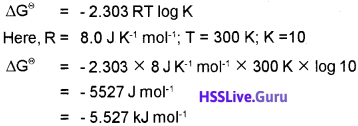
The equilibrium constant for the reaction is 10. Calculate the value of ∆G ; Given R = 8 J K-1 mol-1; T = 300 K.
Answer:
Question 6
Calculate the entropy change in surroundings when 1.0 mol of HzO (I) is formed under standard conditions. Given ∆fH = – 286 kJ mol-1.
Answer:

Question 7.
Comment on the thermodynamic stability of NO(g) and NO2 (g) given :

Answer: