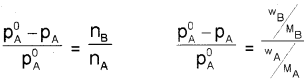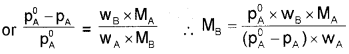Students can Download Chapter 5 Surface Chemistry Notes, Plus Two Chemistry Notes helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus Two Chemistry Notes Chapter 5 Surface Chemistry
Surface chemistry deals with phenomena that occurs at the surfaces or interfaces.
Adsorption:
accumulation of molecular species at the surface rather than in the bulk of a solid or liquid, it is a surface phenomenon, e.g. Moisture gets adsorbed on silica gel.
Adsorbate:
molecular species or substance, which accumulates at the surface.
Adsorbent:
material on the surface of which adsorption takes place, e.g. Charcoal, Silica gel, etc.
Desorption:
process of removing adsorbed substance from the surface of adsorbent.
Difference between Adsorption and Absorption:
Adsorption –
the substance is concentrated only at the surface and does not penetrate to the bulk of the adsorbent.
Absorption –
the substance is uniformly distributed throughout the bulk of the solid, e.g. Moisture gets absorbed on anhydrous CaCl2 while adsorbed on silical gel.

Sorption:
term used when both adsorption and absorption take place simultaneously.
Mechanism of Adsorption :
The unbalanced or residual attractive forces are responsible for attracting the adsorbate particle on adsorbent surface. During adsorption energy decreases, therefore adsorption is exothermic process, i.e., ∆H of adsorption (heat of adsorption) is always negative. The entropy of the system also decreases (∆S = – ve).
Types of Adsorption:
1. Physical Adsorption (Physisorption):
Here the adsorbed molecules are held on the surface of the adsorbent by physical forces such as van der Waals’ forces. It is reversed by reducing pressure or by heating.
Characteristics:
Lack of specificity, easily liquifiable gases readily adsorbed, reversible in nature, extent of adsorption increases with increase in surface area of adsorbent, enthalpy of adsorption quite low (20 – 40 kJ mol’ ).
2. Chemical Adsorption (Chemisorption):
the forces of interaction between the adsorbent and adsorbate are chemical in nature. It cannot be easily reversed.
Characteristics:
High specificity, irreversibility, increases with increase in surface area, enthalpy of adsorption is high (80 -240 kJ mol”1).

Sometimes physisorption and chemisorption occur simultaneously and it is not easy to ascertain the type of adsorption. A physisorption at low temperature may pass into chemisorption as the temperature is increased. For example, dihydrogen is first adsorbed on Ni by van der Waals’forces. Molecules of hydrogen then dissociate to form hydrogen atoms which are held on the surface by chemisorption.
Comparison of Physisorption and Chemisorption
| Physisorption | Chemisorption |
| 1) Arises because of van der Waals’ force | 1) Caused by chemical bond formation |
| 2) Not specific | 2) Highly specific |
| 3) Reversable | 3) Irreversible |
| 4) More easily liquefiable gases are adsorbed readily. | 4) Gases which can react with the adsorbent show chemisorption. |
| 5) Enthalpy of adsorption is low (20-40 kJ mol’1) | 5) Enthalpy of adsorption is high (80-240 kJ mol-1) |
| 6) Low temperature is favourable. It decreases with increase of temperature | 6) Hig temperature is favourable. It increases with increase of temperature |
| 7) No appreciable activation energy is needed. | 7) High activation energy is sometimes needed. |
| 8) Increases with an increase of surface area. | 8) Increases with an increase of surface area. |
| 9) Results into multimolecular layers on adsorbent surface under high pressure. | 9) Results into unimolecular layer |
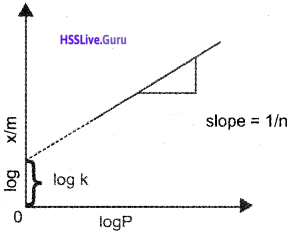
Adsorption Isotherms:
The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm.
Freundlich Adsorption isotherm:
empirical relation between the quantity of gas adsorbed by unit mass of the solid adsorbant and pressure at a particular temperature.
x/m = k.P1/n (n > 1)
x → mass of the gas adsorbed
m → mass of adsorbent
‘k’ and ‘n’ are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
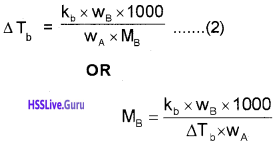
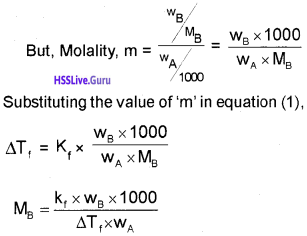
OR log x/m = log k + \(\frac{1}{n}\) log P
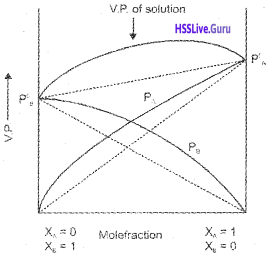
Adsorption from Solution Phase:
Freundlich’s equation approximately describes the behaviour of adsorption from solution.
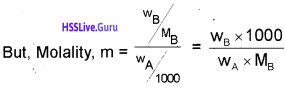
\(\frac{x}{m}\) = k.C1/n m
C – equilibrium concentration
log x/m = log k + \(\frac{1}{n}\) log C
Plotting log x/m vs log C a straight line is obtained
Applications of Adsorption:
Production of high vacuum, in Gas masks – activated charcoal is filled in gas mask to adsorb poisonous gases, for removal of colouring matter from solution in heterogeneous catalysis, in chromatographies analysis, in froth floatation process.
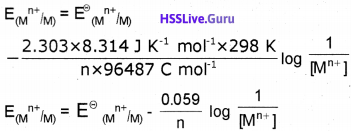
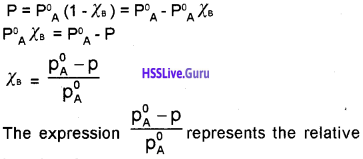
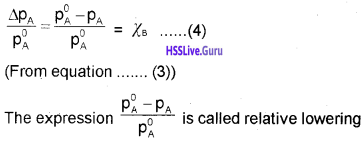
How to find Kp with the entropy and enthalpy amounts and with Gibb’s Free Energy.
Catalysis :
The process of altering the rate of chemical reaction by the addition of a foreign substance (catalyst) is called catalysis, e.g. MnO2 acts as a catalyst in the thermal decomposition of KClO3.

Promoters:
substances that enhance the activity of a catalyst, e.g. In Haber’s process, iron is used as catalyst and molybdenum acts as a promoter.

Poisons:
substances which decrease the activity of a catalyst.
Homogeneous and Heterogeneous Catalysis
a) Homogeneous Catalysis:
When the reactants and catalyst are in the same phase, the process is said to be homogeneous catalysis.
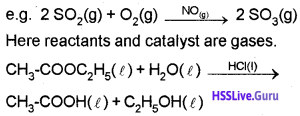
Here both the reactants and the catalyst are in the liquid phase.
Heterogeneous Catalysis:
If the reactants and the catalyst are in different phase, the catalysis known as heterogeneous catalysis.

Here reactants are gaseous state while the catalysts are in the solid state.
Important Features of Solid Catalysts
a) Activity:
ability of catalysts to accelerate a chemical reaction.

But pure mixture of H2 and O2 does not react at all in the absence of a catalyst.
b) Selectivity:
ability of a catalyst to direct a reaction to yield a particular product.
e.g. CO and H2 combine to form different products by using different catalysts.

Shape Selective Catalysis by Zeolites:
The catalytic reaction that depends upon the pore structure of the catalyst and size of the reactant and the product molecules.
Zeolites are good shape-selective catalysts because of their honey comb-like structures. Zeolites are widely used in petrochemical industries for cracking and isomerisation of hydrocarbon.
e.g. ZSM – 5 – which convert alcohols into petrol.
Enzyme Catalysis:
Enzymes are biological catalysts. They catalyse biological reaction in animals and plants to maintain life. e.g.
- Invertase – Cane sugar into glucose and fructose
- Zymase – Glucose into alcohols
- Maltase – Maltose into glucose
- Diastase – Starch into maltose
- Cellulase – Cellulose into glucose
- Urease – Urea into NH3 and CO2
Characteristics:
Highly efficient, highly specific in nature, highly active under optimum temperature, highly active under optimum pH

Mechanism (Lock and key model)
The molecules of the reactant (substrate), which have complementary shape, fit into the cavities on the surface of enzyme particles just like a key fits into a lock. The enzyme catalysed reactions proceeds in two steps:
Step -1 :
Binding of enzyme to sutbstrate to form an activated complex.
E + S → ES*
Step-2 :
Decomposition of the activated complex to form product.
ES* → E + P
Catalysts in Industry
- Finely divided iron with molybdenum as promoter in Haber’s process. (New catalyst: a mixture of iron oxide, potassium oxide and alumina)
- Platinised asbestos in Ostwald’s process
- Platinised asbestos or V205 in Contact process
Colloids:
Heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium, e.g. Starch, Gelatin. In colloids the particle size (diameter) is between 1nm and 1000 nm.
Classification of Colloids:
i) Based on physical state of dispersed phase and dispersion medium:
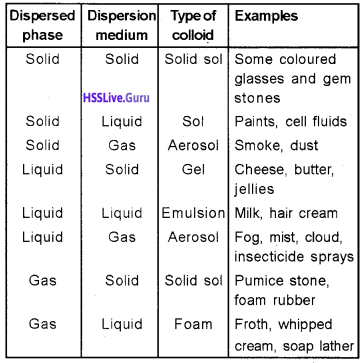
ii) Based on Nature of Interaction between Dispersed Phase and Dispersion Medium:
1. Lyophilic (solvent attracting) Colloids:
there is strong interaction between the dispersed phase and dispersion medium. They are reversible sols. e.g. Starch, gelatin, albumin etc.
2. Lyophobic (solvent repelling) Colloids:
there is little or no interaction between the dispersed phase and dispersion medium. They are also irreversible colloids and are not stable.
iii) Based on Types of Particle of the Dispersed Phase
a) Multimolecular Colloids :
the individual particles consist of an aggregate of atoms or small molecules with molecular size less than 1 nm, the particles are held together by van der Waals’ forces, e.g. Sulphur sol, Gold sol etc.
b) Macromolecular Colloids :
the particles of dispersed phase are sufficiently big in size, maybe in the colloidal range, e.g. Starch, cellulose, proteins.
c) Associated Colloids (Micelles):
colloids which behave as normal strong electrolytes at low concentration but get associated at higher concentrations and behaves as colloidal solutions. The associated particle formed are called micelles.
e.g. Soap, detergents etc.
The formation of micelles take place only above a particular temperature called Kraft temperature (Tk.) and above a particular concentration called Critical Micelle Concentration(CMC).

Mechanism of micelle formation –
In soaps, the RCOO– ions are present on the surface with their COO– groups in water and R staying away from it and remain at the surface. At CMC, the anions are pulled into the bulk of the solution and aggregate to form ‘ionic micelle’ having spherical shape with R pointing towards the centre of the sphere and COO– part remaining outward on the surface of the sphere.
Preparation of Colloids
a) Chemical Methods
Some examples:
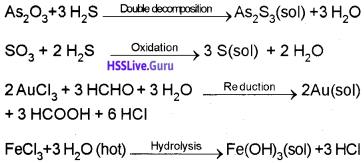
b) Electrical Disintegration or Bredig’s Arc Method
Metallic sols can be prepared by striking an arc between two electrodes of the metal, immersed in the dispersion medium. The metal is vapourised by the arc which then condenses to form particles of colloidal size. e.g. Gold sol, Platinum sol, Silver sol etc.
c) Peptization:
process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of small amount of electrolyte (peptizing agent), e.g. Freshly prepared Fe(OH)3 is peptized by adding small quantity of FeCI3 solution (peptizing agent).

Mechanism of peptization –
During peptization, the precipitate adsorbs one of the ions of the electrolyte on its surface. This causes the development of positive or negative charge on precipitates, which ultimately break up into smaller particles of the size of a colloid.
Purification of Colloids:
process of reducing the amount of impurities to a requisite minimum from the colloids.
i) Dialysis:
process of removing a dissolved substance from a colloid by means of diffusion through a suitable membrane.
ii) Electro-dialysis:
process of dialysis in presence of an applied electric field. It is faster and is applicable if the dissolved substance in the impure colloid is only an electrolyte. The ions present in the colloid migrate out to the oppositely charged electrodes.
iii) Ultrafilteration:
process of separating the colloidal particles from the solvent and soluble solutes present in the colloid by ultra filters. The ultra filter paper is prepared by soaking the filter paper in a colloidion solution (4% solution of nitro cellulose in a mixture of alcohol and ether). It is then hardened by formaldehyde and finally dried.
Properties of Colloids
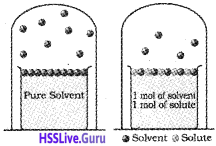
1) Colligative Properties:
values of colligative properties as smaller due to smaller number of particles.
2) Tyndall Effect (Optical Property):
phenomenon of the scattering of light by colloidal particles.
Conditions for observing Tyndall effect:
1. The diameter of the dispersed particles is not much smaller than the wavelength of the light used; and
2. The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude. The ultramicroscope used to observe the light scattered by colloidal particles is based on Tyndall effect.
The colour of the sky can be explained by Tyndall effect. The dust and other colloids present in the atmosphere scatter the light. Only blue light reaches to our eyes.

3) Colour:
It depends on the wavelength of lighty scattered by the dispersed particles which in turn depends on the size and nature of the particles and changes with the manner in which the observer receives the light, e.g. a mixture of milk and water appears blue when viewed by the reflected light and red when viewed by transmitted light.
4) Brownian Movement:
The constant zig-zag movement of the colloidal particles. It is due to the unbalanced bombardment of the particles by the molecules of the dispersion medium. It does not permit the particles to settle and is responsible for the stability of sols. ,
5) Charge on Colloidal Particles:
Colloidal particles carry an electric charge.
Positive charged sols: Al2O3. xH2O, CrO3.xH20, basic dye stuffs, blood (Haemoglobin) etc.
Negatively charged sols:
Metal sols (Cu, Ag, Au), metallic sulphides, acid dyes stuffs, starch, gelatin.
Reason for charge:
It is due to
i) electron capture by sol particles during electrodispersion of metals,
ii) preferential adsorption of ions from solution and/ or
iii) formulation of electrical double layer.
Helmholtz Electrical Double Layer:
combination of two layers of opposite charges around the colloidal particle. The first layer of ions is firmly held and is termed fixed layer while the second layer is mobile which is termed as diffused layer.
Electrokinetic Potential or Zeta Potential:
It is the potential difference between the fixed layer and the diffused layer of opposite changes in the electrical ‘ double layer.
Significance of Charge on Colloidal Particles:
provides stability to the colloid because the repulsive forces between charged particles having same charge prevent them from coalescing or aggregating when they come closer to one another.
6) Electrophoresis:
lled anaphoresis and that of cathode is called cataphoresis.

Coagulation/Flocculation/Precipitation:
process of settling of colloidal particles by the addition of electrolyte.
Coagulation of lyophobic sols can be carried out by the following ways:
Electrophoresis, mutual coagulation (mixing two oppositely charged sols), boiling, persistent dialysis, addition of electrolytes, etc.
Addition of electrolytes –
Colloids interact with ion carrying charge opposite to that present on themselves. This causes neutralisation leading to their coagulation.
Hardy – Schulze Rule:
the greater the valence of the flocculating ion added, the greater is its power to cause precipitation.
The ion having opposite charge to sol particles (coagulating ion) cause coagulation.
In the coagulation of negative sol, the flocculating power is in the order: Al3+ > Ba2+ > Na+
In the coagulation of positive sol, the flocculating power in the order:
[Fe(CN)6]4- > PO43- > SO42-> Cl–
Protective Colloids:
the lyophilic sol used for protection of lyophobic sol.

Emulsions:
liquidin liquid colloidal systems i.e., the dispersion of finely divided droplets in another liquid. There are two types of emulsions.
1) Oil dispersed in water (O/W type):
water acts as dispersion medium, e.g. Milk, Vanishing cream.
2) Water dispersed in oil (W/O type):
oil, acts as dispersion medium.e.g. Butter, Creams, Cod liveroil Emulsification – process of making an emulsion. Emulsion may be obtained by vigourously agitating a mixture of both liquids.
Emulsifying agent or emulsifier –
substance used to stabilise an emulsion. It forms an interfacial film between suspended particles and the medium, e.g.
Emulsifying agents for O/W emulsions :
Proteins, gums, natural and synthetic soaps etc.
Emulsifying agents for W/O emulsions:
Heavy metal salts of fatty acids, long chain alcohols, lampblack etc.

Colloids Around Us :
Fog, mist and rain; food materials, blood, soils, formation of delta.
Application of Colloids
I) In Medicine:
Colloidal medicines are more effective because they have large surface area and are, therefore, easily assimilated, e.g. Colloidal silver (Argyrol) – as eye lotion, Colloidal antimony – in curing Kalaazar, Colloidal gold – for intramuscular injection. Milk of magnesia – in stomach disorder.
II) In industries :
Electrical precipitation of smoke – by Cottrell smoke precipitator, purification of water, tanning, cleansing action of soaps and detergents (micelle formation), photographic plates and films, rubber industry and Industrial products.