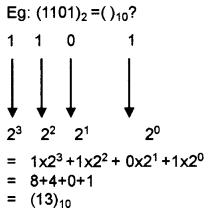
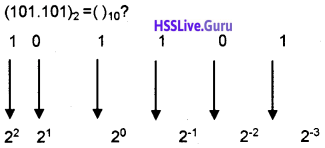
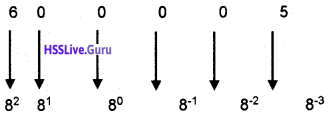
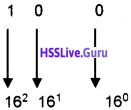
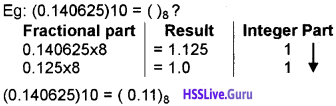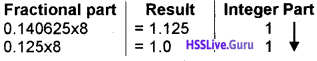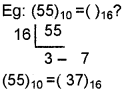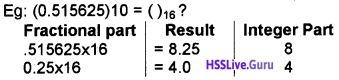Students can Download Chapter 3 Components of the Computer System Notes, Plus One Computer Science Notes helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus One Computer Science Notes Chapter 3 Components of the Computer System
Summary
Hardware:
The tangible parts of a computer that we can touch and see are called hardware. Eg: Monitor, Keyboard, Mouse, CPU, Etc.

Processors:
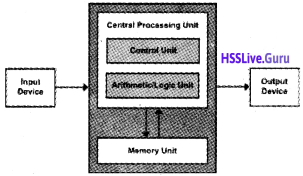
It is the brain of the computer and consists of three components
- Arithmetic Logic Unit(ALU) – As the name implies it performs all calculations and comparison operations.
- Control Unit(CU) – It controls overall functions of a computer
- Registers – It stores the intermediate results temporarily.
A CPU is an Integrated Circuit(IC) package contains millions of transistors and other components. Popular Processors are Intel core i3, core i5, core i7, AMD Quadcore etc.
Important registers inside a CPU are:
- Accumulator: After performing an operation (arithmetic or logical) the result is stored in the accumulator
- Memory Address Register(MAR): It stores the address of memory location to which data is either read or written by the processor.
- Memory Buffer Register (MBR): It stores the data, either to be written to or read from the memory by the processor.
- Instruction Register(IR): It stores the instructions to be executed by the processor.
- Program Counter(PC): It stores the address of the next instruction to be executed by the processor.
Motherboard:
It is a Printed Circuit Board(PCB). All the major components like Processor (Remember the processor must be compatible with the Motherboard), RAM, ROM, HDD, Graphics card, Sound card, etc are connected to the Motherboard.
Peripherals and Ports:
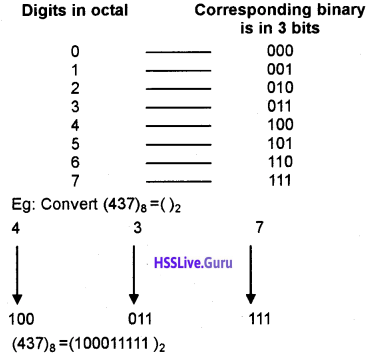
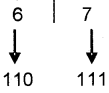
- Serial Port: It transmits data one bit at a time(eg: 101000001010 ). Its transmission speed is low but it is cheaper. It is suitable to transmit data over long distance.
- Parallel port: It can transmit data more than one bit at a time. It is faster and used to connect printer.
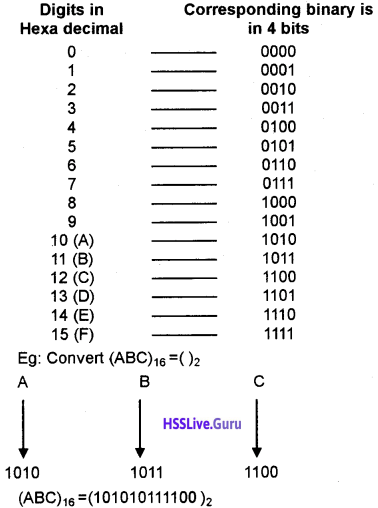
- USB (Universal Serial Bus) port: It has high bandwidth hence it is faster. Nowadays it is used to connect all the devices like keyboard,mouse,printer, scanner, pen drive, digital camera, mobile phones, dongle etc.
- LAN port: By using this port we can connect our computer to another network by a cable.
- PS/2(Personal System/2) port: It is introduced by IBM for connecting keyboard and mouse earlier.
- Audio ports: It is used to connect audio devices like speakers, mic etc.
- Video Graphics Array(VGA) port: It is introduced by IBM to connect monitor or LCD projector to a computer.
- High Definition Multimedia Interface(HDMI): Through this port we can connect high definition quality video and multi-channel audio over a single cable.
Memory:
Storage Unit(Memory Unit): A computer has huge storage capacity. It is used to store data and instructions before starts the processing. Secondly it stores the intermediate results and thirdly it stores information(processed data), that is the final results before send to the output unit(Visual Display Unit, Printer, etc). Memory measuring units are given below.
- 1 bit = 1 or 0(Binary Digit)
- 4 bits = 1 Nibble
- 8 bits = 1 Byte
- 1024 Bytes = 1 KB(Kilo Byte)
- 1024 KB = 1 MB(Mega Byte)
- 1024 MB = 1 GB(Giga Byte)
- 1024 GB = 1 TB(Tera Byte)
- 1024 TB = 1 PB(Peta Byte)
1. Primary Storage alias Main Memory:
It is further be classified into Two – Random Access Memory (FRAM) and Read Only Memory(ROM). The one and only memory that the CPU can directly access is the main memory at a very high speed. It is expensive hence storage capacity is less.
RAM is volatile(when the power is switched off the content will be erased) in nature but ROM is non volatile(lt is permanent). In ROM a “boot up” program called BIOS(Basic Input Output System) is stored to “boots up” the computer when it switched on. Some ROMs are given below.
- PROM(Programmable ROM): It is programmed at the time of manufacturing and cannot be erased.
- EPROM (Erasable PROM): It can be erased and can be reprogrammed using special electronic circuit.
- EEPROM (Electrically EPROM): It can be erased and rewritten electrically
Cache Memory:
The processor is a very high speed memory but comparatively RAM is slower than Processor. So there is a speed mismatch between the RAM and Processor, to resolve this a high speed memory is placed in between these two this memory is called cache memory. Commonly used cache memories are Level(L1) Cache(128 KB), L2(1 MB),L3(8 MB), L4(128MB).
2. Secondary Storage alias Auxiliary Memory:
Because of limited storage capacity of primary memory its need arises. When a user saves a file, it will be stored in this memory hence it is permanent in nature and its capacity is huge. Eg: Hard Disc Drive(HDD), Compact Disc(CD), DVD, Pen Drive, Blu Ray Disc etc.
(i) Magnetic storage device:
It uses plastic tape or metal/plastic discs coated with magnetic material. .
(a) Hard Disk:
Instead of flexible or soft disk it uses rigid material hence the name hard disk. Its storage capacity and data transfer rate are high and low access time. These are more lasting and less error prone. The accessing mechanism and storage media are combined together in a single unit and connect to the motherboard via cable.
(ii) Optical storage device:
(a) Optical Disk:
The high power laser uses a concentrated, narrow beam of light, which is focuses and directed with lenses, prisms and mirrors for recording data. This beams burns very very small spots in master disk, which is used for making molds and these molds are used for making copies on plastic disks.
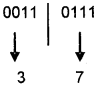
A thin layer of aluminium followed by a transparent plastic layer is deposited on it. The holes made by the laser beam are called pits, interpreted as bit 0 and unburned areas are called lands interpreted as bit 1. Lower power laser beam is used to retrieve the data.
(b) DVD(Digital Versatile Disc):
It is similar to CD but its storage capacity is much higher. The capacity of a DVD starts from 4.7 GB
(c) Blu-ray Disc:
It is used to read and write High Definition video data as well as to store very huge amount of data. While Cd and DVD uses red laser to read and write but it uses Blue-Violet laser, hence the name Blu ray disc. The blue violet laser has shorter wavelength than a red laser so it can pack more data tightly.
(iii) Semiconductor storage (Flash memory):
It uses EEPROM chips. It is faster and long lasting.
- USB flash drive: It is also called thumb drive or pen drive. Its capacity varies from 2 GB to 32 GB.
- Flash memory cards: It is used in Camera, Mobile phones, tablets etc to store all types of data.

Input / Output devices:
It is used to supply: data to the computer for processing
1. Keyboard:
It is the most widely used device to input information in the form of words, numbers etc. There are 101 keys on a standard keyboard. The keys on the keyboard are often classified into alpha numeric keys (A to Z, 0 to 9), function keys (F1 to F12), special purpose keys (Special characters), cursor movement keys (arrow keys). While pressing a key, the corresponding code’s signal is transmitted to the computer.
2. Mouse:
It is a pointing device, that controls the movement of the cursor, or pointer as a display screen. A mouse has two or three buttons, it is often used in GUI oriented computers.
Under the mouse there is a ball, when the mouse moves o.n a flat surface this ball also moves. This mechanical motion is converted into digital values that represents x and y values of the mouse movement.
3. Light Pen:
It is an input device that use a lightsensitive detector to select objects directly on a display screen using a pen. Light pen has a photocell placed in a small tube. By using light pen, we can locate the exact position on the screen.
4. Touch screen:
It allows the user to enter data by simply touching on the display screen. This technology is applied in tablets, cell phones, computers etc.
5. Graphic tablet:
It consists of an electronic writing area. We can create graphical images by using a special pen.
6. Touchpad:
It is a pointing device found on the portable computers(lap top). Just like a mouse it consists of two buttons below the touch surface to do the operations like left click and right click. By using our fingers we can easily operate.
7. Joy Stick:
It is a device that lets the user move an object quickly on the screen. It has a liver that moves in all directions and controls the pointer or object. It is used for computer games and CAD/CAM systems.
8. Microphone:
By using this device we can convert voice signals into digital form.
9. Scanner:
It is used to read text or pictures printed on paper and translate the information into computer usable form. It is just like a photostat machine but it gives information to the computer.
10. Optical Mark Reader (OMR):
This device identifies the presence or absence of a pen or pencil mark. It is used to evaluate objective type exams. In this method special preprinted forms are designed with circles can be marked with dark pencil or ink.
A high intensity beam in the OMR converts this into computer usable form and detects the number and location ofthe pencil marks. By using this we can evaluate easily and reduce the errors.
11. Bar code/Quick Response (QR) code reader:
Light and dark bars are used to record item name, code and price is called Bar Code. This information can be read and input into a computer quickly without errors using Bar Code Readers.
It consists of a photo electric scanner and it is used in super market, jewellery, textiles etc. QR codes are similar to barcodes but it uses two dimensional instead of single dimensional used in Barcode.
12. Biometric sensor:
It is used to read unique human physical features like finger prints, retina, iris pattern, facial expressions etc. Most of you give these data to the Government for Aadhaar.
13. Smart card reader:
A plastic card(may be like your ATM card) stores and transmit data with the help of a reader.

14. Digital Camera:
By using digital camera, we can take photographs and store in a computer. Therefore we can reduce the use of film. Hence it is economical.
Output devices:
After the data processing the result is displayed as soft copy(soft copy can view, only by using a device) or hard copy(lt can read easily).
1. Visual Display Unit:
a. Cathode Ray Tube (CRT):
There are two types of CRT’s, monochrome (Black and white) and colour. Monochrome CRT consists of one electron gun but colour CRT consists of 3 electron guns (Red, Green and Blue) at one end and the other end coated with phosphor. It is a vacuum tube. The phosphor coated screen can glow when electron beams produced by electron guns hit.
It is possible to create all the colours using Red, Green and Blue. The images produced by this is refreshed at the rate of 50 or 60 times each second. Its disadvantage is it is heavy and bulky. It consumes more power and emits heat. But it is cheap. Nowadays its production is stopped by the company.
b. Liquid Crystal Display (LCD):
It consists of two electrically conducting plates filled with liquid crystal. The front plate has transparent electrodes and the back plate is a mirror.
By applying proper electrical signals across the plates, the liquid crystals either transmit or block the light and then reflecting it back from the mirror to the viewer and hence produce images. It is used in where small sized displays are required.
c. Light Emitting Diode(LED):
It uses LED behind the liquid crystals in order to light up the screen. It gives a better quality and clear image with wider viewing angle. Its power consumption is less.
d. Plasma Panels:
It consists of two glass plates filled with neon gas. Each plate has several parallel electrodes, right angles to each other. When low voltage is applied between two electrodes, one on each plate, a small portion of gas is glow and hence produce images.
Plasma displays provide high resolution but are expensive. It is used in, where quality and size is a matter of concern.
e. Organic Light Emitting Diode(OLED) Monitors:
It is made up of millions of tiny LEDs. OLED monitors are thinner and lighter than LCDs and LEDs. It consumes less power and produce better quality images but it is very expensive.
- LCD projector: It is used to display video, images or data from a computer on a large screen. Its main component is a high intensity light producing bulb and a lens.
2. Printer:
There are two types of printers impact and non impact printers. Printers are used to produce hard copy.
Impact Printers:
There is a mechanical contact between print head and the paper.
(i) Dot Matrix Printer:
Here characters are formed by using dots. The printing head contains a vertical array of pins. The letters are formed by using 6 dot rows and 7 dot columns. Such a pattern is called 5 × 7 matrix.
This head moves across the paper, the selected pins fire against an inked ribbon to form characters by dot. They are capable of faster printing, but their quality is not good.
Non-impact Printers:
There is no mechanical contact between print head and paper so carbon copies cannot be possible to take. They are inkjet, laser, thermal printers etc.
(a) Inkjet Printer:
It works in the same fashion as dot matrix printers, but the dots are formed with tiny droplets of ink to be fired from a bottle through a nozzle. These droplets are deflected by an electric field using horizontal and vertical deflection plates to form characters and images.
It is possible to generate colour output. They produce less noise and produce high quality printing output. The printing cost is higher. Here liquid ink is used.

(b)Laser Printer:
It uses photo copying technology. Here instead of liquid ink dry ink powder called toner is used. A drum coated vyith positively charged photo conductive material is scanned by a laser beam.
The positive charges that are illuminated by the beam are dissipated. The drum is then rolled through a reservoir of negatively charged toner which is picked up by the charged portions of the drum.
It adheres to the positive charges and hence creating a page image on the drum. Monochrome laser printer uses a single toner whereas the colour, laser printer uses four toners. Its print quality is good less noise and printing cost is higher.
(c) Thermal Printers:
It is same as dot matrix printer but it needs heat sensitive paper. It produces images by pushing electrically heated pins to the special paper. It does not make an impact on the paper so we cannot produce carbon copies. It produce less noise, low quality print and inexpensive. It is used in fax machine.
3. Plotter:
A plotter is a device that draws pictures or diagrams on paper based on commands from a computer. Plotters draw lines using.a pen. Pen plotters generally use drum or flat bed paper holders. In a drum plotter the paper is mounted on the surface of a drum.
Here the paper is rotated. But in a flat bed plotter the paper does not move and the pen holding mechanism provides the motion that draws pictures. Plotters are used in engineering applications where precision is needed.
4. Three Dimensional (3D) printer:
This device is used to print 3D objects.
5. Audio output devices:
Speakers are used to produce sound by the backward and forward movement of the diaphragm in the speaker according to the electrical signals from the computer through the audio port.
e-Waste(electronic waste):
It refers to the mal functioning electronic products such as faulty computers, mobile phones, tv sets, toys, CFL etc.
Why should we concern about e Waste:
Itcontains poisonous substances such as lead, mercury, cadmium etc and may cause diseases if not properly managed.
What happens to the e Waste:
A small amount is recycled. Due to this our natural resources are contaminated(poisoned). Some of them can recycle properly. But it is a very big problem in front of the Government to collect segregate, recycle and disposal of e-Waste.
e-Waste disposal methods:
- Reuse: Reusability has an important role of e-Waste management and can reduce the volume of e-Waste
- Incineration: It is the process of burning e Waste at high temperature in a chimney
- Recycling of e-Waste: It is the process of making new products from this e-Waste.
- Land filling: It is used to level pits and cover by thick layer of soil.

Students’ role in e-Waste disposal:
- Stop buying unnecessary electronic equipments
- Repair Faulty electronic equipments instead of buying a new one.
- Give electronic equipments to recycle
- Buy durable, efficient, quality, toxic free, good warranty products
- check the website or call the dealer if there is any exchange scheme
- Buy rechargeable battery products
Green computing or Green IT:
It is the study and practice of eco friendly computing or IT such as designing, manufacturing, using and disposal of. computers and components (monitors, printers, storage devices etc.)
Following are some steps to follow to reduce the adverse impact on the global environment
- Turn off computer and other devices when not in use
- Use power saver mode
- Use laptops instead of desktops
- Avoid print outs if not needed
- Use LCD s instead of CRT s to save power
- Use Energy Star rated H/W or S/w and Solar energy(Hybrid Energy)
- Dispose e Waste properly as per norms
Following are the steps to promote green computing:
- Green design: Design energy efficient and eco friendly devices
- Green manufacturing: reduce non eco friendly parts while manufacturing
- Green use: Use energy saver devices
- Green disposal: Use easily disposable devices
Software:
The set of instructions that tell the hardware how to perform a task is called software. Without software computer cannot do anything. Two types System s/w and Application s/w
A. System software: It is a collection of programs used to manage system resources and control its operations. It is further classified into two.
- Operating System
- Language Processor
1. Operating System:
It is collection of programs which acts as an interface between user and computer. Without an operating system computer cannot do anything. Its main function is make the computer usable and use hardware in an efficient manner, eg:- Windows XP, Windows Vista, Linux, Windows 7, etc.
Major functions of an operating System
- Process management: It includes allocation and de allocation of processes(program in execution) as well as scheduling system resources in efficient manner
- Memory management: It.takes care of allocation and de allocation of memory in efficient manner
- File management: This includes organizing, naming, storing, retrieving, sharing , protecting and recovery of files.
- Device management: Many devices are connected to a computer so it must be handled efficiently.
2. Language Processes:
We know that a program is a set of instructions. The instructions to the computer are written in different languages. They are high level language (HLL) and low level language. In HLL English like statements are used to write programs. They are C, C++, COBOL, PASCAL, VB, Java etc. HLL is very easy and can be easily understood by the human being.
Low level language are classified into Assembly Language and Machine Language. In assembly language mnemonics (codes) are used to write programs

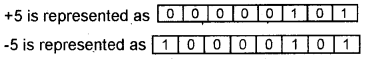
In Machine Language 0’s and 1 ’s are used to write program. It is very difficult but this is the only language which is understood by the computer. Usually programmers prefer HLL to write programs because of its simplicity.
But computer understands only machine language. So there is a translation needed. The program which perform this job are language processors. The different language processors are given below:
- Assembler: This converts programs written in assembly language into machine language.
- Interpreter: This converts a HLL program into machine language by converting and executing it line by line. The first line is converted if there is no error it will be executed otherwise you have to correct it and the second line and so on.
- Compiler: It is same as interpreter but there is a difference it translate HLL program into machine language by converting all the lines at a time. If there is no error then only it will executed.

B. Application Software:
Programs developed to serve a particular application is known as application software. eg: MS Office, Compression Utility, Tally etc. Application software can further be sub divided into three categories.
- Packages
- Utilities
- Customized Software
1. Packages:
Application software that makes the computer useful, for people to do every task. Packages are used to do general purpose application.
They are given below:
(i) Word Processes:
This is used for creation and modification of text document. That means a word processor helps the people to create, edit and format a textual data with less effort and maximum efficiency.
By using word processor we can change font and font size of character, change alignment (left, right, center and justify), check spelling and grammar of the whole document etc. eg: MS Word.
(ii) Spread Sheets:
It contains data or information in rows and columns and can perform calculation (Arithmetic, Relational and logical operation). It helps to calculate results of a particular formula and the formula can apply different cells (A cell is the intersection of a row and column. Each column carries an alphabet for its name and row is numbered).
It is used to prepare budgets, balance sheets, P & L account, Pay roll etc. We can easily prepare graphs and charts using data entered in a worksheet. A file is a work book that contains one or more work sheets, eg: MS Excel is a spread sheet software.
(iii) Presentation and Graphics:
You can present your idea with sound and visual effects with the help of presentation software by preparing slides. The application software that manipulate visual images is known as graphics software. Eg: MS Power Point is a presentation package.
(iv) Data base package:
Data base is a collection of large volume of data. DBMS is a set of programs that manages the datas are for the centralized control of data such that creating new records to the database, deleting, records whenever not wanted from the database and modification of the existing database. Example for a DBMS is MS Access.
DTP Packages:
DTP means Desk Top Publishing. By using this we can create books, periodicals, magazines etc. easily and fastly. Now DTP packages are used to create in Malayalam also, eg: PageMaker.
2. Utilities:
Utilities are programs which are designed to assist computer for its smooth functioning. The utilities are given below.
- Text editor: It is used for creating and editing text files.
- Backup utility: Creating a copy of files in another location to protect them against loss, if your hard disk fails or you accidentally overwrite or delete data.
- Compression Utility: It is used to reduce the size of a file by using a program and can be restored to its original form when needed.
- Disk Defragmenter: It is used to speeds up disk access by rearranging the files that are stored in different locations as fragments to contiguous memory and free space is consolidated in one contiguous block.
- Virus Scanner: It is a program called antivirus software scans the disk for viruses and removes them if any virus is found.
3. Specific purpose software (Customized software):
It is collection of programs which are developed to meet user needs to serve a particular application. It is also called tailor made software.

Free and open source software:
Here “free” means there is no copyright or licensing. That is we can take copies of the s/w or modify the source code without legal permission of its vendor (creator) we can use and distribute its copy to our friends without permission. That is Freedom to use to modify and redistribute.
The Four freedoms are:
- Freedom 0: To run program for any purpose
- Freedom 1: To study how it works and allows you to adapt according to your needs. Also allows to access source code.
- Freedom 2: Allows to take copies and distribute
- Freedom 3: Allows you to change source code and release the program .
Examples for Free and open source software are given below
(i) Linux:
it is a free s/w. It was written by Linux Trovalds at the University of Helsinki. It is a GUI and multi-user, multi-tasking O.S. with many more other features. It is also independent of the hardware. It is used by ISP’s, programmers who uses Java to write program, etc. The main Linux distributors are. Open Linux Red hat, Debian, Gnu Linux, etc.
(ii) GNU/Linux:
It was organized by Richard Stallman in 1983
(iii) GIMP (GNU Image Manipulation Program):
It is a very helpful software to perform all the activities to an image.
(iv) Mozilla Firefox:
This web browser helps the users to browse safely.
(v) OpenOffice.org:
This package contains different s/w s that helps the users to draft letters neatly by using “Writer”, perform calculations by using “Calc” and prepare presentations by using “Impress”. It is platform independent (That means it works on both Linux and Windows platforms.
Freeware:
A s/w with Copy right is available free of cost for unlimited use.
Shareware: It is an introductory pack distributed on a trial basis with limited functionality and period.
![]()
![]()
![]()