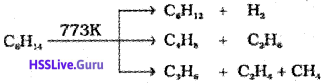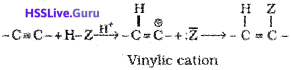Students can Download Chapter 13 Hydrocarbons Notes, Plus One Chemistry Notes helps you to revise the complete Kerala State Syllabus and score more marks in your examinations.
Kerala Plus One Chemistry Notes Chapter 13 Hydrocarbons
Introduction
The compounds formed by carbon and hydrogen are called Hydrocarbons.
Classification
Hydrocarbons are broadly classified into two main classes. Open chain hydrocarbons and closed chain or cyclic hydrocarbons.
Open chain hydrocarbons are hydrocarbons containing open chain of carbon atoms in their molecules. The chain may be straight or branched. These compounds are also called aliphatic hydrocarbons. They are further divided into three classes: Alkanes, alkenes, and alkynes.

Closed chain or cyclic hydrocarbons are hydrocarbons having closed chains or righs in their molecules. They are further divided into two categories: Alicyclic hydrocarbons and aromatic hydrocarbons. Alicyclic hydrocarbons contain ring or closed chain of carbon atoms, but resemble aliphatic compounds in most of their properties. Aromatic hydrocarbons contain at least one benzene ring which is a hexagonal ring of carbon atoms with three double bonds in alternate positions.
ALkanes
Alkanes are saturated aliphatic hydrocarbons containing only carbon-carbon single bonds in their molecules. They can be represented by the general formula cn H2n+2 Where n may have values 1, 2, 3, ………… Since they are relatively inert towards most of the common reagents, they are called paraffins (Latin : parum = Little, affinis = affinity)
Nomenclature
Nomenclature of alkanes has already been discussed in Unit =12 ‘
Preparation:
Petroleum and natural gas are the main sources of alkanes. However, alkanes can be prepared by following methods:
1. From unsaturated hydrocarbons
Dihydrogen gas adds to alkenes and alkynes in the presence of finely divided catalysts like platinum, palladium or nickel to form alkanes. This process is called hydrogenation. These metals adsorb dihydrogen gas on their surfaces and activate the hydrogen – hydrogen bond.
Platinum and palladium catalyse the reaction at room temperature but higher temperature and pressure are required.

2. From alkyl halides
i) Alkyl halides (except fluorides) on reduction with zinc and dilute hydrochloric acid give alkanes.

ii) Alkyl halides on treatment with sodium metal in dry ether (free from moisture) solution give higher alkanes. This reaction is known as Wurtz reaction and is used for the preparation of higher alkanes containing even number of carbon atoms.

3. From carboxylic acids
i) Sodium salts of carboxylic acids on heating with soda lime (mixture of sodium hydroxide and calcium oxide) give alkanes containing one carbon atom less than the carboxylic acid. This process of elimination of carbon dioxide from a carboxylic acid is known as Decarboxylation.

Sodium ethanoate

ii) Kolbe’s electrolytic method:
An aqueous solution of sodium or potassium salt of a carboxylic acid on electrolysis gives alkane containing even number of carbon atoms at the anode.
2CH3COO–Na+ +2H2O
Sodium acetate
-Electrolysis
CH3 – CH3 +2CO2 +H2 +2NaOH
The reaction is supposed to follow the following path:

Methane cannot be prepared by this method.
Properties
Physical properties:
Alkanes are almost non-polar molecules because of the covalent nature of C-C and C-H bonds and due to very little difference of electronegativity between carbon and hydrogen atoms. They possess weak van der Waals’ forces. Due to the weak forces, the first four members, C1 to C4 are gases, C5 to C17 are liquids and those containing 18 carbon atoms or more are solids at 298 K. It is generally observed that in relation to solubility of substances in solvents, polar substances are soluble in polar solvents, whereas the non-polar ones in non-polar solvents i.e., like dissolves like. There is a steady increase in boiling point with increase in molecular mass.

Chemical properties
As already mentioned, alkanes are generally inert towards acids, bases, oxidising and reducing agents. However, they undergo the following reactions under certain conditions.
1. Substitution reactions
One or more hydrogen atoms of alkanes can be replaced by halogens, nitro group, and sulphonic acid group. Halogenation takes place either at higher temperature (573-773 K) or in the presence of diffused sunlight or .ultraviolet light. Lower alkanes do not undergo nitration and sulphonation reactions. These reactions in which hydrogen atoms of alkanes are substituted are known as substitution reactions. As an example,chlorination of methane is given below:
Halogenation
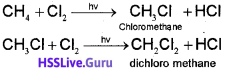
It is found that the rate of reaction of alkanes with halogens is F2 > Cl2 > Br2 > l2. Rate of replacement of hydrogens of alkanes is :3° > 2° > 1 °. Fluorination is too violent to be controlled, lodination is very slow and a reversible reaction. It can be carried out in the presence of oxidizing agents like HIO3 or HNO3.
CH4 +I2 \(\rightleftharpoons \) CH3I+HI
HIO3 +5HI → 3l2 +3H2O
Halogenation is supposed to proceed via free radical chain mechanism involving three steps namely initiation, propagation, and termination as given below:
Mechanism
i) Initiation :
The reaction is initiated by homolysis of chlorine molecule in the presence of light or heat. The Cl-Cl bond isweakerthantheC-C and C-H bond and hence is easiest to break.
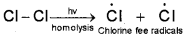
ii) Propagation:
It involves the following steps
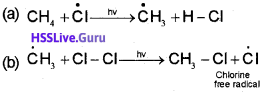
Two such steps given below explain how more highly halogenated products are formed.

iii) Termination :
The reaction stops after some time due to consumption of reactants and / or due to the following side reactions:
The possible chain terminating steps are :

Though in (c), CH3-Cl, the one of the products is formed but free radicals are consumed and the chain is terminated. The above mechanism helps us to understand the reason for the formation of ethane as a byproduct during chlorination of methane.
2. Combustion
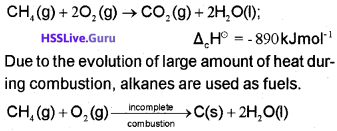
3. Controlled oxidation

iv) Ordinarily alkanes resist oxidation but alkanes having tertiary H atom can be oxidized to corresponding alcohols by potassium permanganate.

4. Isomerisation
n-Alkanes on heating in the presence of anhydrous aluminum chloride and hydrogen chloride gas isomerise to branched-chain alkanes. Major products are given below. Minor products are generally not reported in organic reactions.
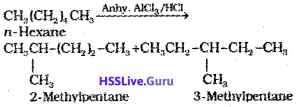
5. Aromatization
n-Alkanes having six or more carbon atoms on heating to 773 K at 10-20 atmospheric pressure in the presence of oxides of vanadium, molybdenum or chromium supported over alumina get dehydrogenated and cyclised to benzene and its homologues. This reaction is known as aromatization or reforming.
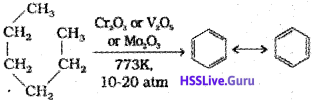
6. Reaction with steam

7. Pyrolysis
Higher alkanes on heating to higher temperature decompose into lower alkanes, alkenes etc. Such a decomposition reaction into smaller fragments by the application of heat is called pyrolysis or cracking.
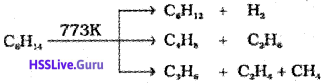
Preparation of oil gas or petrol gas from kerosene oil or petrol involves the principle of pyrolysis.

Conformations
The C-C bonds in alkanes are sigma bonds. Carbon atoms connected through sigma bonds in alkanes can undergo rotation about the bond axis. As a result of this rotation, a large number of different spatial arrangements of atoms of groups attached to the carbon atoms are possible. These different spatial arrangements are called conformations. Thus, the different spatial arrangements of atoms in a molecule which arise due to free rotation about carbon-carbon single bond are called conformations.

Conformations of ethene:
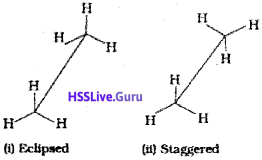
Ethane molecule (C2H6) contains a carbon-carbon single bond with each carbon atom attached to three hydrogen atoms. Considering the ball and stick model of ethane, keep one carbon atom stationary and rotate the other carbon atom around the C-C axis. This rotation results into infinite number of spatial arrangements of hydrogen atoms attached to one carbon atom with respect to the hydrogen atoms attached to the other carbon atom. These are called conformational isomers (conformers). There are two extreme cases. One such confirmation in which hydrogen atoms attached to two carbons are as closed together as possible is called eclipsed conformation and the other in which hydrogens are as far apart as possible is known as the staggered conformation. Any other intermediate conformation is called a skew conformation. lt may be remembered that in all the conformations, the bond angles and the bond lengths remain the same. Eclipsed and the staggered conformations can be represented by Sawhorse and Newman projections.
1. Sawhorse projection
C-C bond is represented by straight line. Upper end of the line is slightly tilted. The front carbon is shown by the lower end of the line and the rear carbon is represented by upper end. The angle between C-H bonds is 120°. Sawhorse projections of eclipsed and staggered conformations are depicted below.
2. Newman projections
In this projection, the molecule is viewed at the C-C bond head on. The carbon atom nearer to the eye is represented by a point. Three hydrogen atoms attached to the front carbon atom are shown by three lines drawn at an angle of 120° to each other. The rear carbon atom (the carbon atom away from the eye) is represented by a circle and the three hydrogen atoms are shown attached to it by the shorter lines drawn at an angle of 120° to each other.
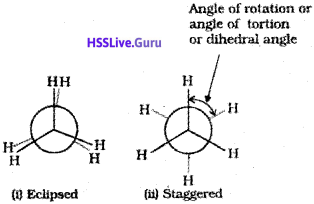
Relative stability of conformations:
As mentioned earlier, in staggered form of ethane, the electron clouds of carbon-hydrogen bonds are as far apart as possible. Thus, there are minimum repulsive forces, minimum energy and maximum stability of the molecule. On the other hand, when the staggered form changes into the eclipsed form, the electron clouds of the carbon-hydrogen bonds come closer to each other resulting in increase in electron cloud repulsions. To check the increased repulsive forces, molecule will have to possess more energy and thus has lesser stability. As already mentioned, the repulsive interaction between the electron clouds, which affects stability of a conformation, is called torsional strain.

Magnitude of torsional strain depends upon the angle of rotation about C-C bond. This angle is also called dihedral angle or torsional angle. Of all the conformations of ethane, the staggered form has the least torsional strain and the eclipsed form, the maximum torsional strain. Thus it may be inferred that rotation around C-C bond in ethane is not completely free. The energy difference between the two extreme forms is of the order of is very small.
Alkenes
Alkenes are unsaturated hydrocarbons containing a carbon-carbon double bond. Acyclic alkenes with one double bond have the general formula CnH2n. Thus they are isomeric with cycloalkanes. They are also known as olefins (Greek word olefiant = oil forming) because the lower members of this family form oily products when treated with chlorine.
We have already seen that a carbon-carbon double bond consists of a sigma bond and a pi-bond. A pi-bond is weaker than a sigma – bond because sidewise overlapping takes place to smaller extent. It should be noted that a double bond is shorter than a single bond. Again, the C-H bond length in ethene is slightly shorter than that in alkanes because it is formed by the overlap of sp² hybrid orbitals of carbon.
Isomerism in Alkenes
Alkenes show chain isomerism, position isomerism and geometrical isomerism,
i) Chain Isomerism :
The simplest alkene which can exhibit isomerism is butene (C4 H8). It exists as two chain isomers.

ii) Position isomerism :
Position isomers of alkenes differ in the position of double bond.

iii) Geometrical isomerism :
The isomers which have the same structural formula but differ in spatial arangement of atoms or groups about the double bond are called geometrical isomers and the phenomenon is known as geometrical isomers of but 2 ene are given below.

The geometrical isomer with similar groups on the same side of the double bond is called the cis isomer while the other isomer with similar groups on opposite side is called the trans isomer. Because of this reason geometrical isomerism is also called cistrans isomerism.

Hindered rotation about carbon-carbon double bond is responsible for the existence of geometrical isomers. As we know, C=C consists of a sigma bond and a pi bond. Due to the presence of pi bond, the groups or atoms attached to doubly bonded carbon atoms cannot rotate about it. They can do so only by breaking the pi-electron cloud.
Preparation
1) From alkynes:
Partially deactivated palladised charcoal is known as Lindlar’s catalyst is used Alkenes thus obtained are having cis geometry. However, alkynes on reduction with sodium in liquid ammonia form trans alkenes.

2) From alkyl halides:
Alkyl halides (R-X)on heating with alcoholic potash eliminate one molecule of halogen acid to form alkenes. This reaction is known as dehydrohalogenation i.e., removal of halogen acid. This is example of β-elimination reaction, since hydrogen atom is eliminated from the β-carbon atom(carbon atom next to the carbon to which halogen is attached).
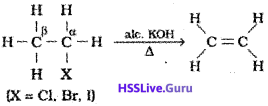
It is observed that for halogens, the rate is:iodine > bromine > chlorine, while for alkyl groups it is: tert > secondary > primary.
3) From vicinal dihalides :
Dihalides in which two halogen atoms are attached to two adjacent carbon atoms are known as vicinal dihalides. Vicinal dihalides on treatment with zinc metal lose a molecule of ZnX2 form an alkene. This reaction is known as dehalogenation.
CH2Br – CH2Br + Zn CH2 = CH2 + ZnBr2

4) From alcohols by acidic dehydration:
Alcohols on heating with concentrated sulphuric acid form alkenes with the elimination of one water molecule. Since a water molecule is eliminated from the alcohol molecule in the presence of an acid, this reaction is known as acidic dehydration of alcohols. This reaction is also the example of β-elimination reaction since-OH group takes out one hydrogen atom from the β-carbon atom.

Chemical Properties
1) Addition of dihydrogen:
Alkenes add up one molecule of dihydrogen gas in the presence of finely divided nickel, palladium or platinum to form alkanes
2) Addition of halogens :
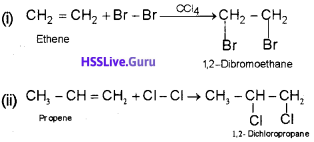
3) Addition of hydrogen halides:
Hydrogen halides (HCl, HBr,HI) add up to alkenes to form alkyl halides. The order of reactivity of the hydrogen halides is HI > HBr> HCI. Like addition of halogens to alkenes, addition of hydrogen halides is also an example of electrophilic addition reaction. Addition reaction of HBr to symmetrical alkenes Addition reactions of HBr to symmetrical alkenes (similar groups attached to double bond) take place by electrophilic addition mechanism.

Addition reaction of HBr to unsymmetrical alkenes (Markovnikov Rule)
There are two possible products in the following reaction
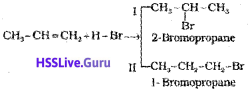
Markovnikov, a Russian chemist made generalisations about such reactions. It to frame a rule called Markovnikov rule. The rule states that negative part of the addendum (adding molecule) gets attached to that carbon atom which possesses lesser number of hydrogen atoms. Thus according to this rule, product I i.e., 2- bromopropane is expected. In actual practice, this is the principal product of the reaction.

Anti Markovnikov addition or peroxide effect or Kharash effect
In the presence of peroxide, addition of HBr to unsymmetrical alkenes like propene takes place contrary to the Markovnikov rule. This happens only with HBr but not with HCI and HI. This addition reaction was observed by M.S. Kharash and F.R. Mayo in 1933. This reaction is known as peroxide or Kharash effect or addition reaction anti to Markovnikov rule.
4) Addition of sulphuric acid:
Cold concentrated sulphuric acid adds to alkenes in accordance with Markovnikov rule to form alkyl hydrogen sulphate by the electrophilic addition reaction.

5) Addition of water :
In the presence of a few drops of concentrated sulphuric acid alkenes react with water to form alcohols, in accordance with the Markovnikov rule.

6) Oxidation:
Alkenes on reaction with cold,dilute, aqueous solution of potassium permanganate (Baeyer’s reagent) produce vicinal glycols. Decolorisation of KMnO. solution is used as a test for unsaturation.

b) Acidic potassium permanganate or acidic potassium dichromate oxidises alkenes to ketones and/or acids depending upon the nature of the alkene and the experimental conditions.

7) Ozonolysis:
Ozonolysis of alkenes involves the addition of ozone molecule to alkene to form ozonide, and then cleavage of the ozonide by Zn- H20 to smaller molecules. This reaction is highly useful in detecting the position of the double bond in alkenes or other unsaturated compounds.
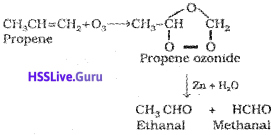
8) Polymerisation:
You are familiar with polythene bags and polythene sheets. Polythene is obtained by the combination of large number of ethene molecules at high temperature, high pressure and in the presence of a catalyst. The large molecules thus obtained are called polymers. This reaction is known as polymerisation. The simple compounds from which polymers are made are called monomers. Other alkenes also undergo polymerisation.

Alkynes
Like alkenes, alkynes are also unsaturated hydrocarbons. They contain at least one triple bond between two carbon atoms. The number of hydrogen atoms is still less in alkynes as compared to alkenes or alkanes. Their general formula is CnH2n-2 Alkynes show chain isomerism and position isomerism.
i) Chain isomerism :
Alkynes having five or more carbon atoms show chain isomerism due to different structures of the carbon chain. For example, the chain isomers of C5H8 are

ii) Position isomerism:
Butyne and higher alkynes show position isomerism. For example, the position isomers of butyne are

Structure Of Triple Bond
Each carbon atom of ethyne has two sp hybridised orbitals. Carbon-carbon sigma (σ)bond is obtained by the head-on overlapping of the two sp hybridised orbitals of the two carbon atoms. The remaining sp hybridised orbital of each carbon atom undergoes overlapping along the internuclear axis with the 1s orbital of each of the two hydrogen atoms forming two C-H sigma bonds. H-C-C bond angle is of 180°. Each carbon has two unhybridised p orbitals which are perpendicular to each other as well as to the plane of the C-C sigma bond. The 2p orbitals of one carbon atom are parallel to the 2p orbitals of the other carbon atom, which undergo lateral or sideways overlapping to form two pi (π) bonds between two carbon atoms. Thus ethyne molecule consists of one C-C σ bond, two C-H σ bonds, and two C-C π bonds. The strength of C ≡ C bond is more than those of C=C bond and C-C bond.
Preparation
1. From calcium carbide:

2. From vicinal dihalides:

Properties
Physical properties
Physical properties of alkynes follow the same trend of alkenes and alkanes. First three members are gases, the next eight are liquids and the higher ones are solids. All alkynes are colourless. Ethyene has characteristic odour. Other members are odourless. Alkynes are weakly polar in nature. They are lighter than water and immiscible with water but soluble in organic solvents like ethers, carbon tetrachloride, and benzene. Their melting point, boiling point and density increase with increase in molar mass.
Chemical Properties
Acidic Character Of Alkyne
A. Acidic character of alkyne:
The acidic character of acetylene and other l-alkynes can be explaned on the basis of the sp hybridisation state of the triply bonded carbon atom. We know that an electron in s-orbital is more tightly held than that in a p-orbital because s-electrons are more close to the nucleus. Now, sp hybridised orbital has greater s-character (50%) as compared to sp² (33%) and sp³ (25%) hybrid orbitals. Due to large s-character, the electrons in sp hybridised orbitals are held more tightly by the nucleus. Thus, sp hybridised carbon is more electronegative than sp² or sp³ hybridised carbon atoms. Due to this, the hydrogen atom attached to sp hybridised carbon atom develops slight positive charge and is acidic in character.
B. Addition reactions:
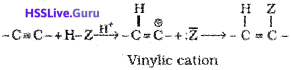
The addition product formed depends upon stability of vinylic cation. Addition in unsymmetrical alkynes takes place according to Markovnikov rule. Majority of the reactions of alkynes are the examples of electrophilic addition reactions. A few addition reactions are given below:
(i) Addition of dihydrogen
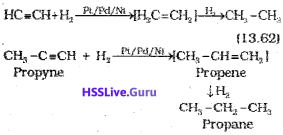
(ii) Addition od halogens

(iii) Addition of hydrogen halides :
Two molecules of hydrogen halides (HCl, HBr,HI) add to alkynes to form gem dihalides (in which two halogens are attached to the same carbon atom)

(iv) Addition of water:

(v) Polymerisation
(a) Linear polymerisation:
Under suitable conditions, linear polymerisation of ethyne takes place to produce polyacetylene or polyethyne which is a high molecularweight polyene containing repeating units . of (CH = CH – CH = CH) and can be represented as —(CH = CH – CH = CH)n — Under special conditions, this polymer conducts electricity.

Thin-film of polyacetylene can be used as electrodes in batteries. These films are good conductors, lighter and cheaper than the metal conductors.
(b) Cyclic polymerisation:

Aromatic Hydrocarbons
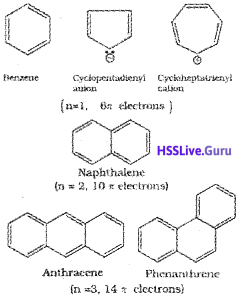
Aromatic compounds are a class of compounds having characteristic stability in spite of having double bonds in their structure. Simple aromatic compounds contain one or more benzene rings. They are known as benzenoid aromatic compounds. There is yet another series of compound which exhibit aromatic properties but lack benzene like structures. They are called non-benzenoid aromatic compounds. Heterocyclic compounds such as pyrrole, thiophene etc. are simple examples of non-benzenoid aromatic compounds. Structures of some simple arenes (benzenoid) are given below.
Resonance and stability of benzene
According to resonance theory, benzene is a resonance hybrid of the two Kekule structures I & II shown below.

These two structures I & II are known as canonical structures of benzene. The actual structure of benzene is intermediate between the two Kekule structures and is referred to as the resonance hybrid. As a result of resonance, all carbon-carbon bond lengths in benzene become equal and lie in between C=C and C-C bond length.

Further, a resonance hybrid will always be more stable than any of the contributing (or canonical) structures. Thus the actual molecule of benzene is more stable than either of the two Kekule structures. The difference between the energy of the most stable contributing structure and the energy of the resonance hybrid is known as resonance energy. Resonance energy of benzene has been found to be about 150 kJ mol-1.
Aromaticity
Benzene was considered as parent ‘aromatic’ compound. Now, the name is applied to all the ring systems whether or not having benzene ring, possessing following characteristics.
i) Planarity
ii) Complete delocalisation of the π electrons in the ring
iii) Presence of (4n + 2)π electrons in the ring where n is an integer (n = 0, 1,2,…).
This is often referred to as Hiickel Rule.
Some examples of aromatic compounds are given below:
Preparation of Benzene
Benzene is commercially isolated from coal tar. However, it may be prepared in the laboratory by the following methods.
i) Cyclic polymerisation of ethyne: (Section 13.4.4)
ii) Decarboxylation of aromatic acids:
Sodium salt of benzoic acid on heating with sodalime gives benzene.
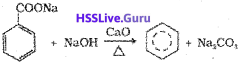
iii) Reduction of Phenol

Properties
Physical Properties
Aromatic hydrocarbons are non- polar molecules and are usually colourless liquids or solids with a characteristic aroma. You are also familiar with naphthalene balls which are used in toilets and for preservation of clothes because of unique smell of the compound and the moth repellent property. Aromatic hydrocarbons are immiscible with water but are readily miscible with organic solvents. They bum with sooty flame.
Chemical properties
Arenes are characterised by electrophilic substitution reactions. However, under special conditions they can also undergo addition and oxidation reactions.

Electrophilic substitution reactions
The common electrophilic substitution reactions of arenes are nitration, halogenation, sulphonation, Friedel Craft’s alkylation and acylation reactions in which attacking reagent is an electrophile (E+)
i) Nitration:

ii) Halogenation:
Arenes react with halogens in the presence of a Lewis acid like anhydrous FeCl3, FeBr3 or AlCl3 to yield haloarenes.

iii) Sulphonation:
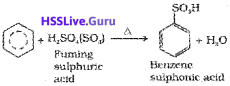
iv) Friedel-Crafts alkylation reaction:

v) Friedel-Crafts acylation reaction:
The reaction of benzene with an acyl halide or acid anhydride in the presence of Lewis acids (AlCl3) yields acyl benzene

If excess of electrophilic reagent is used, further substitution reaction may take place in which other hydrogen atoms of benzene ring may also be successively replaced by the electrophile. For example, benzene on treatment with excess of chlorine in the presence of anhydrous AlCl3 in dark yields hexachlorobenzene (C6Cl6).

a) Generation of electrophile EA :
During chlorination, alkylation and acylation of benzene, an-hydrous AlCl3, being a Lewis acid helps in generation of the elctrophile Cl⊕, R⊕. RC⊕O (acylium ion) respectively by combining with the attacking reagent.

In the case of nitration, the electrophile, nitronium ion, NO2+ is produced by transfer of a proton (from sulphuric acid) to nitric acid in the following manner:

It is interesting to note that in the process of generation of nitronium ion, sulphuric acid serves as an acid and nitric acid as a base.
Thus, it is a simple acid-base equilibrium,
b) Formation of Carbocation (arenium ion):
Attack of electrophile results in the formation of σ – complex or arenium ion in which one of the carbon is sp³ hybridised and the arenium ion gets stabilised by resonance. Sigma complex or arenium ion loses its aromatic character because delocalisation of electrons stops at sp³ hybridised carbon.
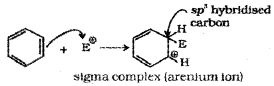
The areneium ion gets stabilised by resonance:

c) Removal of proton:
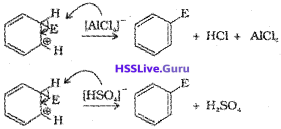
Addition reactions

Combustion:

Directive Influence Of A Functional Group In Monosubstituted Benzene
In benzene all the six hydrogen atoms are equivalent. There fore, replacement of anyone hydrogen atom by a substituent gives a single monosubstituted derivative of benzene. However, when a monosubstituted product is converted into a disubstituted benzene derivative, three different isomers can be obtained. They are,

Carcinogenicity And Toxicity
Benzene and polynuclear hydrocarbons containing more than two benzene ring fused together are toxic and said to posses cancer producing (carcinogenic) property. They are formed due to the incomplete combustion of organic materials like tobacco, coal, and petroleum.